Videos
Browse videos by topic
All Videos
Showing 1-24 of 307 videos

Season 1, Episode 3: Strengthening Safety Oversight for CRO-Sponsor Partnerships
Veeva Systems Inc
@VeevaSystems
Nov 3, 2025
This video provides an in-depth exploration of strengthening safety oversight in CRO-Sponsor partnerships within pharmacovigilance (PV), hosted by David Kološić of Veeva Systems Inc. The discussion features Martijn van de Leur (Chief Commercial Officer) and Olga Asimaki (Head of International QPPV Office and Global Medical Information) from Biomapas, a Contract Research Organization (CRO). The conversation delves into the evolving landscape of PV outsourcing, the critical role of technology like Veeva Volt Safety, the challenges and opportunities presented by automation and Artificial Intelligence (AI), and how Qualified Persons for Pharmacovigilance (QPPVs) are adapting to these shifts while maintaining regulatory compliance. The discussion begins by establishing the importance of CROs in the pharmaceutical industry, particularly in PV, regulatory affairs, medical information, and clinical research. Martijn and Olga share their extensive backgrounds in PV, highlighting the historical context of outsourcing, from early "massive outsourcing projects" with control challenges to the current trend of increased trust and integrated partnerships. They identify key drivers for outsourcing, including cost efficiency, access to specialized expertise, talent attraction and retention, and the need for scalability to adapt to fluctuating resource demands. A significant shift is noted from purely transactional relationships to strategic partnerships where CROs act as an extension of the sponsor's team, offering advisory roles and sharing best practices gleaned from working with diverse clients. A central theme is the adoption of advanced technology to facilitate these partnerships and enhance PV operations. Biomapas's early adoption of Veeva Volt Safety is presented as a case study, driven by the need to replace outdated systems and provide greater transparency and control to sponsors. The speakers emphasize how cloud-based systems like Volt Safety enable real-time visibility, collaborative workflows (e.g., medical review, unblinding in clinical trials), and standardized processes, which are crucial for building trust and ensuring inspection readiness. The conversation then transitions to the QPPV perspective on technology, with Olga stressing that patient safety and regulatory compliance are paramount. She distinguishes between basic automation (like RPAs) and true AI, advocating for a strong foundation of trusted processes and clear oversight before embracing advanced digital transformation, ensuring compliance is not compromised. The latter part of the podcast critically examines the hype surrounding AI in PV. Martijn, with 20 years in the industry, reflects on the slow but significant evolution from paper-based systems to paperless, then to cloud technology, and now to automation. While automation is seen as a tangible "next stage of the revolution," he expresses skepticism about the current state of AI for end-to-end PV automation, citing challenges like data privacy (not being able to mix customer data for training) and the limited data available for smaller clients to train robust AI systems. Olga suggests that current practical AI applications are more likely to be supportive tools, such as in document authoring, content summarization, and generating text based on references, rather than fully autonomous decision-making. Both speakers envision a future (by 2030) where AI-driven signal detection and case processing are seamlessly integrated with real-time data sources, leading to predictive analytics, proactive compliance, and a sustainable, scalable PV system that can manage exponentially growing data volumes. Key Takeaways: * **Evolution of PV Outsourcing:** Outsourcing in pharmacovigilance has evolved from cost-driven, often challenging, large-scale projects to more integrated, trust-based partnerships. This shift is driven by the need for specialized expertise, talent management, and scalable solutions that CROs can provide. * **Strategic CRO-Sponsor Partnerships:** The relationship between CROs and sponsors is moving beyond transactional service provision to a collaborative partnership model. CROs are increasingly seen as an extension of the sponsor's team, offering advisory insights and implementing best practices across multiple clients. * **Transparency and Oversight through Technology:** Modern cloud-based PV systems, such as Veeva Volt Safety, are crucial for fostering trust and transparency in outsourced operations. They provide sponsors with real-time visibility into cases, data, and workflows, allowing for direct involvement in critical steps like medical review and unblinding. * **Standardization as a Key Driver:** The industry is increasingly moving towards standardization in PV processes and system configurations. Utilizing default configurations of validated systems like Veeva Volt Safety simplifies operations, reduces customization costs, and enhances inspection readiness by aligning with health authority expectations. * **QPPV Perspective on Technology Adoption:** For QPPVs, patient safety and regulatory compliance remain the highest priorities. Digital transformation, including automation and AI, must be built upon a strong foundation of trusted processes, data visibility, and clear oversight to ensure compliance is never compromised. * **Distinguishing Automation from AI:** It's important to differentiate between basic automation (e.g., RPAs) and advanced AI in PV. While automation is already delivering practical benefits, true AI for end-to-end PV processes is still in its early stages and requires careful validation and quality control. * **AI's Current Practical Applications:** Currently, AI is more realistically implemented as a supportive tool in PV, particularly for tasks like document authoring, generating content summaries from references, and assisting with text creation, rather than fully replacing human decision-making. Human review and approval remain essential. * **Challenges for AI in PV:** Significant hurdles for widespread AI adoption include data privacy concerns (inability to pool data from multiple clients for training) and the lack of sufficient, diverse data from individual small clients to effectively train robust AI models. * **Regulatory Lag and Uncertainty:** The pace of technological advancement often outstrips regulatory evolution, creating uncertainty for QPPVs. Clear, concrete guidance from regulatory authorities is needed to enable the industry to confidently lean into innovation without compromising compliance. * **Continuous Validation and Proactive Change Management:** Validation of PV systems and tools should not be a one-time event but a continuous process, adapting to evolving needs and configurations. Proactive change management is vital to ensure successful implementation and adoption of new technologies. * **Future Vision for PV (2030):** The future of PV is envisioned as a seamless integration of AI-driven signal detection and case processing with trusted, real-time data sources. This will lead to connected, intelligent safety systems capable of predictive analytics, anticipating compliance needs, and supporting data-driven decisions. * **Technology as a Necessity for Scalability:** With increasing case volumes and pressure to control costs, technology is no longer a "nice-to-have" but a "must" for PV departments. It is essential for achieving scalability, sustainability, and operational efficiency without exponential cost increases. Tools/Resources Mentioned: * **Veeva Volt Safety:** A cloud-based safety database system used by CROs and pharmaceutical companies for pharmacovigilance operations. Key Concepts: * **Pharmacovigilance (PV):** The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. * **CRO-Sponsor Partnerships:** Collaborative relationships between Contract Research Organizations (CROs) and pharmaceutical/biotech sponsors, particularly in outsourcing specialized services like pharmacovigilance. * **QPPV (Qualified Person for Pharmacovigilance):** A designated individual within a marketing authorization holder (or CRO acting on their behalf) responsible for the establishment and maintenance of the pharmacovigilance system. * **Automation (RPA):** The use of technology to perform tasks with minimal human intervention, often referring to Robotic Process Automation (RPA) for repetitive, rule-based tasks. * **Artificial Intelligence (AI) / Large Language Models (LLMs):** Advanced computational systems designed to simulate human intelligence, including learning, reasoning, and problem-solving. In PV, this includes potential applications for data analysis, signal detection, and document generation.

CEOs Control $1.3 Trillion in Healthcare Spending for 165 Million Americans
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Nov 2, 2025
This video provides an in-depth exploration of the profile and psychology of CEOs who control a significant portion of America's healthcare spending, specifically for employer-sponsored health plans. Dr. Eric Bricker, the speaker, begins by establishing the immense financial responsibility these CEOs bear, overseeing $1.3 trillion in healthcare spending for 165 million Americans. He highlights that while this responsibility is often acknowledged, the specific characteristics and motivations of these decision-makers are frequently overlooked. The presentation aims to equip viewers with a practical understanding of how to effectively engage and persuade CEOs to improve healthcare outcomes, emphasizing that understanding "who these people are" is crucial for driving change. The video then delves into a detailed demographic and psychological profile of the typical American CEO. Demographically, CEOs are predominantly male (80%), white (90%), college graduates (98%), with an average age of 51-52, and a significant portion (57%) play golf. Politically, a majority (55%) lean Republican based on donation patterns. Psychologically, using the Five Factor Model of Personality, CEOs score high on Openness (indicating they are risk-takers and open to new ideas), high on Conscientiousness (action-oriented, organized, and focused on getting things done), and high on Extroversion (outgoing). Conversely, they score low on Agreeableness (tending to be self-centered and less empathetic to others' problems) and low on Neuroticism (meaning they are generally unemotional and stable). Building on this profile, Dr. Bricker outlines a strategic approach to persuasion using the ancient Greek framework of Ethos, Pathos, and Logos. For Ethos (credibility), he suggests understanding and engaging with their interests, such as golf, and maintaining high enthusiasm, as extroverts dislike low-energy interactions. For Pathos (empathy), the advice is to appeal directly to their financial self-interest, demonstrating how healthcare improvements will save their business money and increase its valuation (e.g., through EBITDA multiples), and to consider the impact on their immediate family. Crucially, he warns against broad appeals to "fixing healthcare in America" or emotional stories of employee suffering, as these do not resonate with the typical CEO's low agreeableness. Finally, for Logos (logic), the recommendation is to present rational arguments backed by hard numbers and to paint a clear, positive vision for their company's future, leveraging their openness to new ideas and risk-taking nature. Key Takeaways: * **CEOs as Key Decision-Makers:** CEOs are ultimately responsible for the budgetary and policy decisions regarding employer-sponsored health plans, controlling $1.3 trillion in spending for 165 million Americans. Understanding their profile is essential for anyone seeking to improve healthcare within this segment. * **Demographic Profile of CEOs:** The typical CEO is 80% male, 51-52 years old, 90% white, 98% college-educated, 88% married, and 57% play golf. Politically, 55% tend to support Republicans. * **Personality Traits (Big Five Model):** CEOs exhibit high Openness (risk-takers, open to new ideas), high Conscientiousness (action-oriented, organized), high Extroversion (outgoing), low Agreeableness (self-centered, less empathetic), and low Neuroticism (unemotional). * **Ethos (Credibility) Strategy:** To build credibility, understand and engage with their interests, such as golf culture (even if not playing, be present in the milieu). Additionally, always project enthusiasm and high energy, as extroverted CEOs tend to dislike low-energy interactions. * **Pathos (Empathy) Strategy:** Appeal to the CEO's direct financial self-interest by clearly demonstrating how proposed healthcare changes will save their business money, improve profitability, and increase business valuation (e.g., through higher EBITDA multiples). * **Focus on Immediate Family:** CEOs often make decisions based on how health plan changes will impact their immediate family (spouse and children), which can be a more effective emotional appeal than broader concerns for employees. * **Avoid Generic Appeals:** Do not attempt to persuade CEOs with broad, emotional appeals about "fixing healthcare in America" or stories of general employee suffering, as their low agreeableness means these messages are unlikely to resonate. * **Logos (Logic) Strategy:** Present arguments with strong, rational evidence backed by hard numbers and data. CEOs are analytical and require concrete proof for any proposed changes. * **Paint a Vision for the Future:** Leverage their high openness and risk-taking nature by presenting a clear, positive vision for their company's future with the proposed changes, rather than focusing solely on current problems. * **Quantify Financial Impact:** Translate healthcare cost savings into direct impacts on business valuation. For example, a $3 million reduction in healthcare costs could increase a company's sale value by $15 million (based on a 5x EBITDA multiple). * **"Business is Show Business":** Maintain a professional and enthusiastic demeanor, even on challenging days, as this aligns with the extroverted nature of many CEOs. * **Strategic Networking:** Being present in environments where CEOs congregate, such as golf courses or charity events, can be a highly effective strategy for building relationships and generating leads. Tools/Resources Mentioned: * **Zippia.com:** Cited as a source for CEO demographic data. * **Wiley Online Library, NBER, Benefitspro.com, GAO, AEAweb, Verywellmind.com:** Various research and professional publications referenced for CEO data and personality traits. * **The Big Five Factor Model of Personality:** A psychological framework used to describe CEO personality traits (Openness, Conscientiousness, Extroversion, Agreeableness, Neuroticism). * **Ethos, Pathos, Logos:** An ancient Greek rhetorical framework for persuasion, applied to interacting with CEOs. * **John Torres, CEO of Saragraph:** Mentioned as a successful example of a CEO who kept health plan costs flat for nine years. * **"The Companies That Solved Healthcare":** A book written by John Torres. * **"16 Lessons in the Business of Healing":** A book by Dr. Eric Bricker. Key Concepts: * **Employer-Sponsored Health Plans:** Healthcare coverage provided by employers to their employees and dependents. * **Big Five Factor Model of Personality:** A widely accepted model describing five broad dimensions of personality: Openness to Experience, Conscientiousness, Extraversion, Agreeableness, and Neuroticism. * **Ethos, Pathos, Logos:** Three modes of persuasion identified by Aristotle: Ethos (appeal to credibility), Pathos (appeal to emotion), and Logos (appeal to logic). * **EBITDA (Earnings Before Interest, Taxes, Depreciation, and Amortization):** A measure of a company's financial performance, often used in business valuation where a company's sale price is a multiple of its EBITDA. Examples/Case Studies: * **John Torres, CEO of Saragraph:** Successfully managed to keep his company's health plan costs flat for nine consecutive years, demonstrating effective leadership in healthcare cost management. * **Business Valuation Impact:** The video illustrates how a reduction in healthcare costs, such as $3 million, can significantly increase a company's overall valuation (e.g., by $15 million if the business is valued at a 5x EBITDA multiple), directly appealing to a CEO's financial self-interest.

We Played "Guess the Pharma Ad" and Failed Miserably
Self-Funded
@SelfFunded
Oct 31, 2025
This video features a game titled "Guess the Pharma Ad," where hosts attempt to identify direct-to-consumer (DTC) pharmaceutical drugs based solely on their visual commercials, with all drug information obscured. The primary purpose of the segment is to highlight the often-confusing, abstract, and seemingly disconnected nature of pharmaceutical advertising, particularly the reliance on generic "B-roll" footage rather than direct depictions of conditions or drug effects. The hosts, Nathaniel Smith and Kyle Minick, react with amusement and frustration as they try to match vague visuals to specific medications, ultimately demonstrating how difficult it is for even informed viewers to discern the purpose of these high-budget advertisements. Throughout the game, the discussion naturally pivots to the staggering costs associated with these advertised drugs. As each commercial's true identity is revealed, the annual out-of-pocket costs for medications like Eylea, Ponvori, Nerdt ODT, Renvoke, and Skyrizi are disclosed, ranging from tens of thousands to over a hundred thousand dollars annually. This financial revelation underscores the significant economic implications of these drugs for patients and healthcare systems. The hosts also touch upon the specific indications for each drug, noting how broad some of them are (e.g., Skyrizi for multiple inflammatory conditions) and the often tenuous link between the ad's narrative and the drug's therapeutic target. The video progresses through five distinct commercials, each prompting guesses and commentary on the ad's production value, narrative, and perceived target demographic. From a ballet performance to anime sequences and celebrity endorsements, the diverse advertising styles are critiqued for their lack of clarity and direct relevance to the conditions they treat. The segment concludes with a reveal of how poorly the participants performed in the guessing game, reinforcing the central thesis that DTC pharma ads are inherently confusing and often fail to clearly communicate their message, despite their high production costs and the critical nature of the products they promote. Key Takeaways: * **Prevalence of DTC Pharma Advertising:** Direct-to-consumer pharmaceutical commercials are ubiquitous, particularly in media targeting older demographics (e.g., NASCAR broadcasts), indicating a significant investment by pharmaceutical companies in this marketing channel. * **Abstract and Confusing Ad Content:** Many DTC pharma ads rely heavily on generic "B-roll" footage (e.g., happy families, nature scenes) that bears little to no direct relation to the drug's indication or mechanism of action, making it difficult for viewers to understand what the product is for. * **High Production Values:** Despite their often vague messaging, these commercials frequently boast high production values, including elaborate animation (e.g., a French anime commercial for Ponvori) and celebrity endorsements (e.g., Lady Gaga for Nerdt ODT), suggesting substantial marketing budgets. * **Staggering Drug Costs:** The annual out-of-pocket costs for many specialty drugs advertised are exceptionally high, with examples including Eylea ($10,000), Nerdt ODT ($13,000-$20,000), Renvoke ($17,000-$30,000), Ponvori ($84,000-$100,000), and Skyrizi ($120,000-$240,000). * **Broad Indications for Specialty Drugs:** Several high-cost drugs, such as Renvoke and Skyrizi, are approved to treat multiple conditions (e.g., rheumatoid arthritis, Crohn's disease, ulcerative colitis, psoriasis), positioning them as multi-purpose solutions for inflammatory and autoimmune disorders. * **Challenges in Patient Education:** The abstract nature of these commercials presents a significant challenge for patient education, as the ads often fail to clearly communicate the drug's purpose, benefits, or the specific condition it treats, potentially leading to confusion or misinformed inquiries. * **Impact of Rebates on Drug Pricing:** The discussion briefly highlights how rebates, often negotiated through Pharmacy Benefit Managers (PBMs), can significantly reduce the net cost of a drug, impacting the actual out-of-pocket expense for patients versus the list price. * **Demographic Targeting:** Ads are often tailored to perceived demographic interests, such as basketball for an arthritis medication, although the connection to the actual drug's benefit can still be tenuous. * **Regulatory Compliance Implications (Implicit):** While not explicitly discussed, the confusing nature of these ads implicitly raises questions about how pharmaceutical companies balance creative marketing with regulatory requirements for clear and non-misleading communication about drug efficacy and safety. * **Opportunity for AI in Marketing Analysis:** The observed disconnect between ad content and drug purpose suggests an opportunity for AI and LLM solutions to analyze the effectiveness of pharmaceutical commercial operations, potentially identifying patterns in successful patient engagement or optimizing content for clarity and compliance. Key Concepts: * **Direct-to-Consumer (DTC) Pharma Advertising:** Pharmaceutical companies marketing prescription drugs directly to the public through mass media. * **B-roll:** Supplemental or alternate footage intercut with the main shot, often generic or illustrative, used to establish a mood or provide visual context without direct narrative. * **Specialty Drugs:** High-cost, high-complexity medications often used to treat chronic, rare, or complex conditions, typically requiring special handling, administration, or monitoring. * **Indications:** The specific conditions or diseases for which a drug is approved and prescribed. * **Annual Cost (Out-of-Pocket):** The estimated yearly expense a patient might incur for a medication, often before insurance or rebates. Examples/Case Studies: * **Eylea:** An eye disease medication (e.g., age-related macular degeneration) with an annual cost of approximately $10,000. Its commercial featured a grandmother watching ballet. * **Ponvori:** A multiple sclerosis (MS) treatment, costing $84,000-$100,000 annually. Its ad was a high-production-value French anime sequence. * **Nerdt ODT:** A drug for treating and preventing migraines, with an annual cost of $13,000-$20,000. Featured Lady Gaga in its commercial. * **Renvoke:** Treats rheumatoid arthritis, Crohn's disease, and ulcerative colitis, costing $17,000-$30,000 annually. Its commercial showed men playing basketball. * **Skyrizi:** Treats rheumatoid arthritis, psoriasis, Crohn's disease, and ulcerative colitis, with an annual cost of $120,000-$240,000 (before rebates). Its ad featured a music teacher.

A deep dive into Veeva AI for Life science - Interview with Josh Callan and Manuel Mitola
ctcHealth Consulting, Manuel Mitola, AI for Pharma
/@AIForPharma
Oct 15, 2025
This video directly discusses Veeva AI, its integration with Veeva applications (including CRM), and its application within the pharmaceutical and life sciences industries. This video explores Veeva's recent launch of Veeva AI, delving into its unique value proposition, technical architecture, and strategic implications for the life sciences industry. Josh Callan, Head of Strategy for Southern Europe at Veeva, explains how Veeva AI is built directly into the core of life science-specific Vault applications, enabling it to understand business rules, context, and access all Veeva data, documents, and workflows. The discussion highlights the transformative potential of generative AI for automating tasks, improving productivity, and enhancing content personalization for field teams like MSLs and sales reps. A significant portion addresses critical concerns around compliance, reliability, and data security, outlining Veeva's approach to preventing hallucinations, ensuring auditability, and maintaining data privacy through a "bring your own LLM" model. The interview also touches upon common challenges in AI adoption, such as the lack of strategic vision, poor data foundations, and the need for effective change management, concluding with an overview of Veeva's AI Partner Program designed to foster ecosystem integration. Key Takeaways: * **Integrated & Life Science-Specific AI:** Veeva AI is deeply embedded within Veeva's Vault platform, leveraging existing business rules, context, and data to provide life science-specific AI applications and use cases, differentiating it from generic AI solutions. * **Compliance, Reliability, and Auditability:** Veeva addresses concerns about AI hallucinations and black-box outcomes through strict data grounding, continuous quality and auditing processes, and built-in safety filters to ensure outputs are accurate, high-quality, and compliant with industry regulations. * **Customer-Controlled Data Security & Flexibility:** Veeva's "bring your own LLM" model allows customers to run their chosen LLM within their own secure cloud environment, retaining full control over data processing and security, while also offering flexibility in selecting the most suitable LLM based on performance, cost, or regional compliance needs. * **Actionable AI Agents for Commercial Operations:** Flagship AI agents like the CRM bot are designed to proactively assist field teams (e.g., MSLs, sales reps) by recommending content, suggesting actions, summarizing HCP preferences, and even enabling direct actions like scheduling calls, significantly boosting productivity and HCP engagement quality. * **Critical Challenges to AI Adoption:** Successful AI implementation in life sciences faces hurdles including a lack of strategic vision (tech-first mindset), the necessity of a robust data foundation ("garbage in, garbage out"), and effective change management to ensure end-user adoption. * **Ecosystem-Driven Partner Strategy:** The Veeva AI Partner Program provides technology, support, and training (including access to the Vault direct data API and sandboxes) to enable partners to seamlessly integrate their AI solutions with the Vault platform, fostering a broader ecosystem of innovation.

Veeva Vault CRM Review & Demo | What to Know Before Buying
How to Hippo 🦛
/@HowToHippopotamus
Oct 6, 2025
This video provides a detailed review and demo of Veeva Vault CRM, an enterprise application specifically designed for the life sciences industry, including pharmaceutical, biotech, and medical device companies. It highlights how the platform helps streamline operations, ensure regulatory compliance, and enhance engagement with healthcare providers (HCPs). The discussion covers the core functionalities of Veeva Vault CRM, including its central customer relationship management module for managing HCP interactions and field rep activities, a Campaign Manager for compliant multi-channel marketing, a Service Center for handling medical information requests and customer support, and a Patient CRM focused on patient engagement and support programs while maintaining HIPAA compliance. The video also touches upon Veeva's broader ecosystem, mentioning Development, Quality, Medical, and Commercial Clouds, and notes the inclusion of AI-powered features. It advises potential buyers to consider the platform's specialized design for the regulatory environment and customer needs of the life sciences sector. Key Takeaways: * Veeva Vault CRM is an enterprise solution tailored for pharmaceutical, biotech, and medical device companies, integrating CRM functionalities with deep compliance and regulatory management for HCP engagement. * The platform offers a comprehensive suite of modules including a core CRM for managing HCP interactions, a Campaign Manager for compliant marketing, a Service Center for medical information requests, and a Patient CRM for patient engagement and support. * Veeva Vault is part of a larger ecosystem, encompassing Development, Quality, Medical, and Commercial Clouds, indicating its extensive capabilities across various life sciences operations. * The system is positioned as a next-generation CRM, incorporating AI-powered features to enhance commercial operations and other functions. * Pricing for Veeva Vault CRM is not publicly disclosed and requires direct consultation with Veeva sales, reflecting its enterprise-level, tailored solution approach. * Companies in the life sciences industry should consider Veeva Vault CRM for its specialized understanding of regulatory environments and customer needs, while those outside this niche may find it less suitable.

Season 1 Episode 6: Improving the Customer Experience through Medical-Commercial Collaboration
Veeva Systems Inc
@VeevaSystems
Oct 3, 2025
This video explores the critical importance of medical-commercial collaboration within the pharmaceutical industry to enhance the customer (HCP) experience and drive better patient outcomes. Industry leaders from UCB and Takeda discuss the challenges of traditional silos, the strategic imperative for integrated efforts in product launches, and the pivotal role of technology, data, and AI in fostering a more unified and effective future for healthcare engagement. Key Takeaways: * **Essential Cross-Functional Collaboration:** Effective medical-commercial-clinical collaboration is paramount for successful product launches, optimizing HCP engagement, and ultimately improving patient outcomes. A lack of coordination leads to inefficiencies, missed opportunities, and confusion among healthcare providers. * **Technology as an Integration Enabler:** Platforms like Veeva CRM, Veeva Vault, Veeva Link Key People, and Veeva Link Workflow are crucial for creating transparency around HCP engagements, centralizing approved medical content, and facilitating coordinated cross-functional planning. * **AI Augments, Data Foundations Enable:** While AI, particularly Generative AI, is recognized as a powerful tool to augment medical affairs by optimizing tasks and enhancing insight detection, a robust data foundation (including data governance, standards, and management) is critical for its successful implementation and for deriving actionable insights. * **Change Management is Key to Adoption:** Successful implementation of integrated processes and technologies requires strong senior leadership buy-in, early and continuous engagement with all stakeholders (including compliance and legal), and a phased approach to manage change fatigue and ensure end-user adoption. * **Measuring Impact through Data:** Increased adoption of integrated systems provides the necessary data to measure operational effectiveness, engagement quality, and insights gathering, which are foundational steps towards ultimately assessing and improving medical affairs' impact on patient outcomes and clinical practice.

Season 4 Episode 2: Patient vs Process Bridging the Gap for a Better Trial Experience
Veeva Systems Inc
@VeevaSystems
Oct 1, 2025
This video provides an in-depth exploration of bridging the gap between patient experience and clinical trial processes, emphasizing a patient-centric approach. Hosted by Manny Vazquez, Senior Director of Clinical Data Strategy at Veeva, the episode features Joyce Moore, a leading voice in patient recruitment with 25 years of industry experience, most recently at Allucent. The discussion highlights the critical shift from viewing patients merely as subjects to seeing them as collaborators, underscoring the importance of understanding their lives and challenges outside the clinical setting. The conversation delves into how patient engagement and site engagement are intrinsically linked, asserting that one cannot truly thrive without the other. Joyce Moore shares her journey from traditional patient recruitment to embracing decentralized trials (DCTs) and Electronic Clinical Outcome Assessment (eCOA) technologies, all driven by the goal of making trial participation easier for patients. She explains her team's role at Allucent in defining sponsor problems, developing patient-resonant materials, conducting digital outreach, and working with patient advocacy groups. A significant portion of the discussion focuses on the burden placed on both patients and sites by increasingly complex protocols, advocating for technology solutions that seamlessly integrate into existing workflows without adding undue stress. The speakers also explore the ethical implications of data collection, questioning the necessity of extensive exploratory endpoints and advocating for an "endpoint-driven design" that focuses on critical data. They discuss the potential of digital endpoints as exploratory measures to pave the way for more patient-centric trials in the future, while acknowledging the need for regulatory acceptance and clear communication with patients. The concept of "immemorable" technology for sites is introduced, suggesting that the best technology is one that is so intuitive and integrated that site staff barely notice they are using it. The episode concludes with a powerful call to action for the industry to engage patients earlier, simplify protocols, and prioritize sharing data and trial progress back with participants. Key Takeaways: * **Patient-Centricity is Paramount:** Patients should be viewed as collaborators, not just subjects. Understanding their daily lives, challenges, and motivations is crucial for successful engagement and retention in clinical trials. * **Interconnectedness of Patient and Site Engagement:** Effective patient engagement cannot occur without robust site engagement. Supporting sites and reducing their burden directly translates to a better experience for patients. * **Technology for Seamless Integration:** Clinical trial technology, such as eCOA, must be designed to integrate smoothly into existing site workflows and SOPs. The goal is for technology to be "immemorable," meaning it's so intuitive that site staff don't even notice they're using it, allowing them to focus on patient care. * **Addressing Trial Complexity and Burden:** The increasing complexity of clinical trial protocols places significant burden on both sites and patients. This includes long site visits, extensive travel, and the impact on patients' families, which can turn a short appointment into an all-day event. * **Protocol Optimization is Essential:** There is a critical need for protocol optimization to reduce unnecessary data collection. Focusing on "endpoint-driven design" ensures that only data essential for proving the hypothesis is collected, potentially reducing patient and site burden. * **Ethical Data Collection:** The ethics of collecting extensive exploratory endpoints, especially if their future use is uncertain, should be carefully considered. Every data point collected from a patient should have a clear purpose and value. * **The Value of Digital Endpoints:** Digital endpoints, even when initially exploratory, are vital for gathering data that can lead to more patient-centric clinical trials and monitoring in the future, potentially replacing traditional, burdensome assessments. * **Transparent Communication with Patients:** Explaining the "why" behind data collection and trial procedures to patients can significantly improve compliance and engagement. Treating patients like adults who understand the purpose of their participation fosters trust. * **Strategic Decentralized Trial (DCT) Implementation:** While DCTs aim to reduce patient burden, the specific implementation (e.g., centralized home health vs. site-led home visits) needs to consider patient and site preferences. Patients, especially in pediatric or elderly populations, may prefer familiar site staff visiting their homes. * **Early Patient Community Engagement:** Engaging patient communities as early as possible in the protocol design phase allows for true input, leading to lighter, more patient-friendly protocols that better reflect their needs and realities. * **Returning Data to Patients:** The industry has a responsibility to give patients their data back, both personal health information and updates on trial progress. This reciprocates their significant contribution and provides valuable insights into their own health and the study's impact. * **Just-in-Time Training for Sites:** Overburdening sites with extensive training should be avoided. "Just-in-time" training, delivered precisely when needed, is a more effective and less burdensome approach for site staff who are primarily focused on patient care. * **Cost-Benefit Analysis of Data Points:** Attaching a value or cost to each data point can serve as ammunition for sponsors to critically evaluate the necessity of collecting certain information, potentially streamlining protocols and reducing overall trial costs. **Key Concepts:** * **eCOA (Electronic Clinical Outcome Assessment):** Technology used to collect patient-reported outcomes, clinician-reported outcomes, or observer-reported outcomes electronically, often via devices like tablets or smartphones. * **DCT (Decentralized Clinical Trials):** Clinical trials where some or all trial-related activities occur at locations other than traditional clinical sites, such as a patient's home, using technology for remote monitoring and data collection. * **Endpoint-Driven Design:** A methodology for designing clinical trial protocols that prioritizes the collection of only the data necessary to prove the primary and critical secondary endpoints, thereby reducing unnecessary data points and associated burden. * **Patient Burden vs. Site Burden:** The cumulative physical, emotional, and logistical challenges faced by patients participating in a trial versus the operational and administrative challenges faced by clinical trial sites. * **Digital Endpoints:** Objective, quantifiable physiological and behavioral measures collected by connected digital health technologies (e.g., wearables, sensors) that are relevant to a patient's health status. **Examples/Case Studies:** * **Father of a child with a rare disease:** A personal anecdote illustrating how a two-hour site appointment could translate into an 8-10 hour day for a patient and their family due to travel, preparation, and logistical challenges, highlighting the significant patient burden. * **Elderly patient population and home health:** An example where sites expressed concern about centralized nurses visiting their elderly patients, preferring to send their own known staff. This underscores the importance of trust and established relationships in home health settings within DCTs.

Interview with Christina Brennan SVP Clinical Research at Northwell at Veeva R&D and Quality Summit
Moe Alsumidaie
/@Annexclinical
Sep 29, 2025
This video directly addresses critical operational challenges within clinical research, a core area of the pharmaceutical and life sciences industries. The discussion extensively covers the role of technology, AI, and data engineering in optimizing clinical trial operations, particularly patient recruitment, while also touching upon regulatory compliance and the need for efficient, integrated software solutions. This video explores persistent operational bottlenecks in clinical trials, with a strong emphasis on patient recruitment challenges and the transformative role of technology and AI in addressing them. Dr. Christina Brennan, SVP Clinical Research at Northwell, discusses the need to leverage electronic medical records (EHRs) and advanced AI techniques like Natural Language Processing (NLP) to move beyond simple diagnosis codes and accurately identify eligible patients based on complex inclusion/exclusion criteria. The conversation also highlights the critical need for greater site input in protocol design to ensure feasibility and alignment with standard of care, thereby reducing protocol deviations. A significant theme is the increasing burden on study coordinators due to a proliferation of disparate technology platforms, leading to burnout, and the importance of implementing workload acuity tools to manage responsibilities effectively. Finally, the discussion touches on the evolving sponsor-site relationship, emphasizing communication and partnership, and cautiously explores the potential for AI agents to assist with protocol-related inquiries while underscoring the necessity of human oversight. Key Takeaways: * **AI and NLP for Enhanced Patient Recruitment:** There is a significant opportunity to utilize AI and Natural Language Processing (NLP) to analyze detailed EHR notes, moving beyond basic diagnosis codes to precisely identify patients meeting complex clinical trial eligibility criteria, thereby addressing a major bottleneck in trial timelines. * **Mitigating Technology-Induced Coordinator Burnout:** While technology is essential, the sheer number of unintegrated platforms often creates more work for study coordinators. Sites and sponsors must prioritize technologies that genuinely reduce administrative burden and streamline workflows, rather than adding new layers of complexity. * **Criticality of Site-Centric Protocol Design:** Sponsors often overlook valuable site input during protocol development, leading to designs that are impractical or misaligned with standard of care. Early and consistent engagement with sites, particularly for schedule of assessments and through revived investigator meetings, is crucial for designing feasible and compliant trials. * **Workload Acuity for Staff Retention:** To combat study coordinator burnout and maintain study quality, sites should implement workload acuity tools. These tools must consider not just the number of trials, but also their complexity, screening effort, and ongoing regulatory demands to ensure fair and sustainable workload distribution. * **Human Oversight in AI-Powered Clinical Support:** While AI agents show promise for assisting with protocol-related questions and data access (e.g., in line with E6R3 principles), human oversight remains paramount. In clinical research, where patient safety and regulatory compliance are critical, AI should function as a tool to augment, not replace, human expertise and accountability.

Staying Ahead of Compliance – Automating Metadata Change Detection in Veeva Vault Recording
FocalCXM
/@focalcxm
Sep 26, 2025
This video discusses the critical challenge of managing metadata changes within Veeva Vault in the highly regulated life sciences industry. It highlights how even minor, undocumented alterations to metadata—such as picklist values, object configurations, or attributes—can lead to significant compliance risks, audit findings, and operational inefficiencies. The speakers from Focal CXM present an automated solution designed to proactively detect, track, and report these metadata changes, moving away from time-consuming and error-prone manual monitoring. The solution leverages Veeva APIs to capture real-time metadata, compare it against established baselines, and identify discrepancies, thereby enhancing visibility and strengthening internal governance in line with FDA, EMA, and GxP guidelines. Demos illustrate how this can be implemented using a data flow platform and AWS Lambda, with a vision for future integration of agentic AI for enhanced control. Key Takeaways: * **Criticality of Metadata Governance:** Undocumented metadata changes in regulated environments like Veeva Vault pose substantial compliance and audit risks, impacting workflows, user experience, and training across the life sciences enterprise. * **Automated Compliance Monitoring:** The presented solution automates the detection, tracking, and reporting of metadata changes using Veeva APIs, allowing for continuous monitoring and proactive identification of potential issues, such as changes in picklist values or object attributes. * **Broad Applicability and Extensibility:** The underlying methodology for baseline comparison and change detection is applicable not only to Veeva Vault but also to other enterprise systems like Salesforce, and can be extended to broader use cases such as enterprise data reconciliation and data quality profiling. * **Foundation for Trustworthy Data:** The speakers emphasize that establishing trust in enterprise data through robust data quality and reconciliation processes is fundamental, serving as the essential groundwork for effective AI and agentic AI applications. * **Proactive Risk Mitigation:** By providing immediate visibility into metadata updates, the automated system helps regulatory, quality, and IT teams proactively flag risks related to MLR workflows, security roles, document states, and compliance-critical fields, reducing manual effort and anxiety.

The $30 Billion SaaS Company You’ve Never Heard of
Bret Larsen
/@brtlrsn
Sep 17, 2025
This video explores the strategic journey of Veeva, a $30 billion SaaS company that achieved immense success by adopting a highly specialized approach within the pharmaceutical industry. The speaker details how Veeva's founder, Peter Gasner, identified a critical unmet need in pharma for industry-specific software, particularly for CRM, compliance, and regulated workflows, at a time when major companies were still relying on spreadsheets. Veeva's "monk strategy" involved ignoring venture capital trends, focusing exclusively on one vertical, and building a product that deeply understood the nuances and high stakes of the life sciences sector. Initially built on Salesforce's platform, Veeva strategically evolved to develop its own comprehensive "Vault" platform, expanding beyond CRM to encompass document management, clinical trials, regulatory approvals, and manufacturing quality, thereby becoming an indispensable infrastructure for modern medicine. The video emphasizes Veeva's disciplined, profitable business model and its ability to build an unassailable moat by embedding itself across the entire pharmaceutical lifecycle. Key Takeaways: * **Power of Vertical Specialization:** Veeva's success demonstrates the profound impact of extreme focus on a single, complex, and highly regulated industry (pharmaceuticals), addressing its unique challenges rather than pursuing broad market appeal. * **Industry-Specific Solutions:** The initial product, a custom-built CRM for life sciences sales reps, included critical features like sample tracking, compliance checks, and regulated workflows that generic CRMs could not provide, directly meeting the industry's specific needs. * **Strategic Platform Evolution:** Veeva leveraged Salesforce's platform for initial speed and credibility but strategically developed its own "Vault" platform to gain greater control, flexibility, and margin, ultimately achieving independence and owning its technology stack. * **Holistic Industry Infrastructure:** Beyond CRM, Veeva expanded its offerings into document management, clinical trial systems, regulatory approvals, and manufacturing quality, embedding itself as a mission-critical operating system across the entire pharmaceutical product lifecycle. * **Disciplined Business Model:** Veeva achieved significant growth and profitability with minimal venture capital, prioritizing lean operations and a disciplined approach over rapid, cash-burning expansion, proving that sustainable growth can be achieved without aggressive fundraising. * **Addressing Regulatory Complexity:** A core element of Veeva's value proposition was its ability to build software that inherently understood and streamlined the stringent compliance, regulatory, and GxP requirements of the pharmaceutical industry.

Veeva Vault Safety Training | Individual & Corporate Training | Vistasparks Solutions
Vistasparks Solutions
/@Vistasparks-Solutions
Sep 17, 2025
This video provides an in-depth exploration of Vistasparks Solutions' training program for Veeva Vault Safety, a critical cloud-based platform for managing safety data and ensuring regulatory compliance within the pharmaceutical industry. The presentation emphasizes the program's role in enhancing organizational compliance, fostering excellence, and equipping professionals with the necessary skills for pharmacovigilance, drug safety, and regulatory adherence. It outlines the comprehensive nature of the training, catering to both individual professionals seeking career advancement and corporate teams aiming for streamlined, compliant operations. The training program is structured to cover the fundamentals of Veeva Vault Safety, its advantages, and best practices. Key themes include end-to-end safety case management, regulatory compliance, reporting, audit readiness, and the integration of Veeva Vault Safety with other Veeva modules and enterprise systems. The video highlights the platform's importance as an industry standard for global GXP compliance, managing case intake, adhering to ICH E2B standards for efficient processing, and simplifying regulatory submissions. Advanced safety analytics and reporting capabilities are also discussed, providing real-time insights for quicker decision-making and maintaining regulatory compliance. Vistasparks Solutions offers flexible learning formats, including self-paced modules, virtual classes with real-time interaction, and on-the-job training, all accessible via their Veeva Education Services portal. For corporate clients, the training is customized, role-based, and includes features like real-time tracking, comprehensive dashboards, automated retraining, and integration with Veeva QualityDocs. The video presents case studies, such as Kaioa Kirin Inc., which achieved faster qualification and improved audit readiness through unified management, and Pepkin, which scaled compliance training for its growing teams, demonstrating the tangible benefits of the program in improving efficiency and compliance. Key Takeaways: * **Veeva Vault Safety's Critical Role:** The platform is essential for pharmaceutical organizations to efficiently manage safety data, processes, and ensure compliance with strict industry regulations, including global GXP standards and ICH E2B for adverse event reporting. * **Comprehensive Training Scope:** The training covers an introduction to Veeva Vault Safety, end-to-end safety case management, regulatory compliance, reporting, audit readiness, role-based access, workflows, automation, and integration with other Veeva Vault modules. * **Ensuring Regulatory Compliance:** Training familiarizes employees with the platform's features and ensures they stay updated with safety regulations, thereby avoiding potential legal and financial consequences for the company and fostering a culture of safety. * **Industry Standard Adoption:** Veeva Vault Safety is positioned as the preferred solution for global GXP compliance, trusted by over 20 of the world's top pharmaceutical companies, including Pfizer, Novartis, and Roche, for mission-critical safety operations. * **Advanced Analytics and Reporting:** The program emphasizes utilizing advanced safety analytics and reporting tools to gain real-time insights, enabling quicker decisions, maintaining regulatory compliance, and supporting audit readiness with metrics and role performance analytics. * **Role-Based Learning Paths:** Customized learning paths are offered for individuals, including "Vault Platform Primer" (technical foundations), "Technical Foundations" (hands-on practice and administration), and "Clinical Operations" (integrating eTMF and CTMS for clinical safety). * **Flexible Learning Formats:** Vistasparks provides self-paced learning modules, virtual classes with real-time interaction and practical exercises, and on-the-job training, catering to diverse learning styles and schedules. * **Corporate Training Benefits:** Corporate programs offer customized, role-based curricula, real-time tracking through dashboards, automated retraining, and integration with Veeva QualityDocs to streamline training management and enhance organizational compliance. * **Demonstrated Success via Case Studies:** Examples like Kaioa Kirin Inc. highlight improved qualification processes and enhanced audit readiness, while Pepkin's case showcases scalable compliance training, improved employee engagement, and training efficiency metrics. * **Benefits of Integration and Automation:** The training covers features like auto-assignment of tasks, lifecycle management for training tasks within Veeva Vault, and document update triggers that automatically sense changes in quality management system (QMS) documents, ensuring compliance and accuracy. * **Career Advancement Opportunities:** The training helps individuals earn industry-recognized credentials, validate expertise in Veeva Vault Safety, enhance technical skills, boost confidence in leading CRM migration, and prepare for new certification exams (e.g., Q3 2025 exam mentioned). * **Continuous Learning and Support:** The program emphasizes continuous learning to stay updated with the latest features and advancements, supported by expert trainers, 24/7 access to materials, and ongoing assistance for queries and project guidance. Tools/Resources Mentioned: * Veeva Vault Safety * Veeva Vault (general platform) * Veeva CRM (mentioned in context of career growth) * Veeva QualityDocs * Veeva Education Services portal * eTMF (Electronic Trial Master File) * CTMS (Clinical Trial Management System) * Learning X solutions (mentioned in Pepkin case study) Key Concepts: * **Pharmacovigilance:** The science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. * **Drug Safety:** The processes and systems in place to monitor, detect, assess, and prevent adverse effects of pharmaceutical products. * **Regulatory Compliance:** Adherence to laws, regulations, guidelines, and specifications relevant to the pharmaceutical and life sciences industry, including GXP (Good Practices) and ICH E2B standards for electronic transmission of individual case safety reports. * **Adverse Event Management:** The systematic process of collecting, assessing, and reporting adverse events related to pharmaceutical products. * **Safety Data Management:** The efficient and compliant handling of all data related to drug safety, from collection to reporting and analysis. * **Audit Readiness:** The state of being prepared for regulatory inspections and audits, with all documentation and processes in order. * **Role-based Training:** Customized training programs designed to equip individuals with specific knowledge and skills relevant to their roles and responsibilities within an organization. * **Advanced Safety Analytics:** Utilizing data analysis tools to gain deeper insights into safety data, identify trends, and support proactive decision-making. * **Integration and Automation:** Connecting different systems and automating tasks (e.g., auto-assignment, document update triggers) to improve efficiency, reduce errors, and ensure compliance. Examples/Case Studies: * **Kaioa Kirin Inc.:** This pharmaceutical company faced challenges with fragmented document and training systems. By implementing Vistasparks Solutions' unified management in an integrated Veeva system, they achieved significant improvements in their qualification process and enhanced audit readiness, leading to faster qualification and simplified GXP training delivery. * **Pepkin:** This client needed to scale compliance training for its growing teams. By implementing Veeva training and Learning X solutions from Vistasparks, Pepkin achieved scalable compliance training that met their expansion needs, resulting in improved employee engagement and training efficiency metrics. * **Leading Pharmaceutical Companies:** The video states that over 20 of the world's top pharmaceutical companies, including Pfizer, Novartis, and Roche, rely on Veeva Vault Safety for their mission-critical safety operations, underscoring its status as an industry standard.

Season 4 Episode 1: Biggest Risks (and Possible Rewards) of AI in Clinical Data
Veeva Systems Inc
/@VeevaSystems
Sep 15, 2025
This podcast episode explores the significant risks and potential rewards of integrating AI into clinical data management and development, featuring insights from Veeva's CTO, a clinical digital innovation leader at Bayer, and a consulting partner specializing in technology adoption. The discussion centers on identifying pragmatic, real-world applications of AI that deliver tangible value, emphasizing the critical human-machine relationship within a regulated environment. Key topics include leveraging AI for efficient data review, document generation, and the challenges of transitioning from deterministic to non-deterministic AI models. Key Takeaways: * AI's immediate value in clinical data lies in accelerating tasks that require extensive review and pattern detection, such as automated audit trail review (e.g., for ICH GCP R3 compliance), document consistency, and query management, enabling proactive quality improvement. * The integration of non-deterministic AI, like LLMs, into regulated clinical processes requires a "human in the loop" approach, where AI provides suggestions and insights, but human oversight maintains accountability and builds trust, especially given the potential for varied outputs from the same inputs. * Successful AI adoption demands a focus on identifying clear business value and solving specific problems, rather than merely pursuing "cool" technologies. Prioritizing initiatives that offer significant benefit and can be realistically implemented within reasonable timeframes is crucial. * Standardization, particularly of foundational elements like clinical protocols (e.g., through digital protocols and standards like CDISC), is essential for AI to achieve transformative efficiencies, such as a "zero-week study build" for ECRF and data cleaning rules. * Regulatory scrutiny will increasingly require formal risk assessments for AI applications, especially concerning quality control, managing AI hallucination, and justifying the removal of human oversight in automated processes. * The industry exhibits a paradox in change management: reluctance for established operational improvements versus an eager, sometimes uncritical, embrace of new AI technologies, highlighting a need for pragmatic and structured experimentation. * Ultimately, AI in clinical trials should aim to simplify existing complex layers, benefit patients, and optimize study processes, rather than merely adding more complexity, necessitating a strategic re-evaluation of current methodologies.

America's Most Powerful Hospitals... The Game Show
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Sep 14, 2025
This video, presented in a "game show" format by Dr. Eric Bricker of AHealthcareZ, provides a critical overview of America's most powerful hospital systems across the 10 largest metropolitan areas. The core purpose is to equip employee benefits professionals, HR leaders, and CFOs with the knowledge necessary to understand where their healthcare spending is directed and to empower them to engage directly with healthcare providers. Dr. Bricker emphasizes that knowing these dominant hospital systems is as fundamental for employers as knowing major sports teams for a football fan, given the substantial financial outlay involved. The video systematically goes through the top 10 largest U.S. metropolitan areas, starting with New York City and progressing to Phoenix, identifying the approximate population and the leading hospital systems within each. For instance, New York City features Northwell, Mount Sinai, New York Presbyterian, and NYU, while Los Angeles includes Kaiser, Providence, Common Spirit, and Cedar Sinai. This segment highlights the regional fragmentation and concentration of power within the hospital sector, underscoring that employers often deal with a diverse set of powerful providers across different geographies. The speaker's approach is to make this often-dry financial information engaging and memorable through the game show structure. Beyond simply listing powerful hospital systems, the video's central thesis is a call to action for employers to bypass traditional insurance carriers and establish direct relationships with healthcare providers. Dr. Bricker argues that employers, as the ultimate payers, possess significant leverage ("the gold") and can dictate terms. He cites examples of large corporations like Disney and eBay, and even smaller employers, successfully negotiating direct contracts with major hospital systems or specialized facilities like ambulatory surgery centers (ASCs), endoscopy centers, and imaging centers. The underlying message is that the current carrier-centric system is not working efficiently, and proactive employer engagement can lead to "win-win" relationships and better deals for employees and their families. Key Takeaways: * **Identify Major Healthcare Spenders:** Employers, particularly employee benefits professionals, must be intimately familiar with the largest and most powerful hospital systems in the metropolitan areas where their employees reside, as these are the primary recipients of their healthcare spending. * **Regional Dominance:** The video highlights that powerful hospital systems are highly regionalized; knowing the dominant players in one major city (e.g., New York) does not translate to another (e.g., Los Angeles), necessitating a broad understanding of the national landscape. * **Top 10 Metro Areas & Key Systems:** The presentation details the major hospital systems in the 10 largest U.S. metropolitan areas, including New York City (Northwell, Mount Sinai), Los Angeles (Kaiser, Providence), Chicago (Advocate, Northwestern), Dallas-Fort Worth (Baylor Scott & White, Texas Health Resources), Houston (Memorial Hermann, Houston Methodist), Miami (Baptist, Jackson), DC-Baltimore (Inova, MedStar), Atlanta (Emory, Piedmont), Philadelphia (UPenn, Thomas Jefferson), and Phoenix (Banner, Common Spirit). * **Employer Leverage ("The Gold"):** Employers hold significant financial power as the ultimate payers in the healthcare system, a position that grants them the ability to influence terms and negotiate directly with providers. * **Direct Contracting is a Best Practice:** Forward-thinking employer-sponsored health plans are increasingly adopting direct contracting with hospital systems and other healthcare providers as a strategy to optimize costs and improve care quality, bypassing traditional insurance carriers. * **Precedent for Direct Deals:** Examples like Disney's direct arrangement with Orlando Health, eBay's with a Salt Lake City hospital system, and a Dallas furniture retailer's local agreements demonstrate the feasibility and benefits of direct employer-provider relationships. * **Accessibility for Smaller Employers:** Even smaller employers, who might not have the negotiating power for major hospital systems, can pursue direct contracts with specialized providers such as ambulatory surgery centers, endoscopy centers, and imaging centers, which are often eager to offer special deals outside of insurance networks. * **Provider Willingness for Direct Deals:** Many healthcare providers, especially smaller and specialized centers, are keen to work directly with employers, as it allows them to avoid the administrative complexities and reduced reimbursements often associated with insurance carriers. * **Critique of Carrier-Centric Model:** The video implicitly critiques the current system where employers rely solely on insurance carriers, suggesting that this model is "not working" and advocating for a more proactive, direct approach to healthcare purchasing. * **Actionable Advice: Build Relationships:** Employers are encouraged to move beyond simply writing "blank checks" to providers via carriers and instead establish direct relationships, fostering common ground for "win-win" outcomes.

No Surprises Act Independent Dispute Resolution Drives Up Healthcare Costs in America
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Sep 7, 2025
This video provides an in-depth exploration of the unintended consequences of the No Surprises Act (NSA) Independent Dispute Resolution (IDR) process on employer-sponsored health insurance and overall healthcare costs in America. Dr. Eric Bricker begins by reviewing the original intent of the NSA, which was enacted approximately four to five years ago to protect patients from receiving unexpected "out-of-network" bills when they sought care at "in-network" facilities. This typically occurred with ancillary services like radiology, emergency room physicians, anesthesiologists, pathologists, and surgical assistants who might be out-of-network even if the hospital itself was in-network. The NSA mandated that instead of billing the patient, the insurance carrier and the physician group must resolve payment disputes directly. The core issue arises when the insurer and provider cannot agree on a payment amount, leading them to the federal government's IDR process, which functions as a "baseball-style" arbitration. In this system, both parties submit their "best offer," and the arbitrator must choose one offer in its entirety, rather than splitting the difference. The video highlights a significant volume of these disputes, with approximately 300,000 cases per quarter, a number that has been steadily increasing. Alarmingly, providers (physician groups) win 80-85% of these cases. The financial impact is substantial: providers are winning amounts that average 4.5 times the Qualified Payment Amount (QPA), which is typically the median in-network reimbursement rate. Specific examples cited include radiologists winning 6 times the QPA and neurosurgeons winning 11 times the QPA, translating to significantly higher multiples of Medicare rates. One extreme case mentioned was a surgical assistant receiving a $200,000 payment for a single operation through the IDR process, directly contributing to an employer's budget overrun. Dr. Bricker further explains that a relatively small number of physician groups, predominantly those owned by private equity firms (such as Radiology Partners, TeamHealth, and SCP), are driving the majority of these IDR cases. He asserts that these private equity-owned entities are solely focused on maximizing profits and have no interest in lowering healthcare costs, effectively exploiting the IDR process to increase reimbursements. The video also scrutinizes the arbitrators themselves, who are outsourced contractors to the federal government. There are about 15 such contractors, and their provider win rates vary dramatically, from as high as 90% to as low as 33%, with the majority leaning towards higher provider favoritism. This variability suggests that parties strategically select arbitrators known to be favorable to their side. The speaker concludes by offering actionable advice for self-funded employers to mitigate these rising costs, emphasizing their fiduciary responsibility to plan members. Key Takeaways: * **No Surprises Act's Unintended Consequences:** While designed to protect patients from surprise out-of-network bills, the NSA's Independent Dispute Resolution (IDR) process has inadvertently led to a significant increase in healthcare costs for employer-sponsored insurance due to inflated provider reimbursements. * **IDR Process Mechanics:** The IDR process is a "baseball-style" arbitration where an independent arbitrator chooses one of two offers (from the insurer or the provider) without compromise, leading to binary, often high-value, outcomes. * **High Provider Win Rates and Inflated Payments:** Providers win 80-85% of IDR cases, securing payments that average 4.5 times the Qualified Payment Amount (QPA), which is the median in-network rate. This significantly exceeds standard in-network rates. * **Exorbitant Reimbursement Examples:** Specific instances include radiologists winning 6 times the QPA, neurosurgeons winning 11 times the QPA, and a surgical assistant receiving $200,000 for a single operation through IDR, directly impacting employer budgets. * **Private Equity's Role in Cost Escalation:** A small number of private equity-owned physician groups (e.g., Radiology Partners, TeamHealth, SCP) are aggressively utilizing the IDR process, driven by a profit motive that actively contributes to rising healthcare costs. * **Geographic Concentration of IDR Activity:** The majority of IDR cases and associated high costs are concentrated in specific states, identified as hotspots for provider fraud: Texas, Florida, New Jersey, New York, Arizona, Tennessee, and Georgia. * **Arbitrator Transparency and Bias Concerns:** There are approximately 15 federal contractors acting as arbitrators, with highly variable provider win rates (some as high as 90%). This lack of transparency and potential for bias warrants investigation, as parties may strategically select arbitrators. * **Employer Fiduciary Responsibility:** Self-funded employers have a fiduciary responsibility to their plan members to be good stewards of their health plans, which includes actively monitoring and addressing the financial impact of the IDR process. * **Actionable Advice: Demand Claims Data:** Employers should demand that their insurance carriers identify claims that have gone through the IDR process to quantify costs and pinpoint "offending" provider groups and associated hospitals. Carriers may initially resist providing this data. * **Actionable Advice: Steer Employees Away:** Employers can implement plan designs, direct primary care referrals, and direct contracting strategies to steer plan members away from hospitals and facilities that employ out-of-network provider groups known for high IDR wins. * **Actionable Advice: Lobby for Congressional Oversight:** Employers should contact their congressional representatives to advocate for public hearings and investigations by the Department of Justice and CMS into the arbitrators and the IDR process, questioning the appropriateness of the lopsided judgments. * **Disparity in Provider Financial Health:** The video highlights that while some rural hospitals may be struggling, certain provider groups, particularly in specific states, are "raking it in" through these processes, indicating a significant financial disparity within the provider landscape. * **Systemic Exploitation:** The speaker emphasizes that the current IDR system is being exploited by certain entities, and without proactive measures from self-funded employers and government oversight, this exploitation and the resulting increase in healthcare costs will continue. Key Concepts: * **No Surprises Act (NSA):** Federal legislation designed to protect patients from unexpected medical bills from out-of-network providers at in-network facilities. * **Independent Dispute Resolution (IDR):** The arbitration process established by the NSA for resolving payment disputes between insurance carriers and out-of-network providers when they cannot agree on a payment amount. * **Qualified Payment Amount (QPA):** The median in-network rate for a service, often used as a benchmark in payment negotiations and IDR cases. * **Self-funded Employer:** An employer that directly assumes the financial risk for providing healthcare benefits to its employees, rather than purchasing health insurance from a third-party carrier. Examples/Case Studies: * **Specific Reimbursement Multiples:** Radiologists receiving 6 times the QPA; neurosurgeons receiving 11 times the QPA. * **High-Cost Surgical Assistant Claim:** A single surgical assistant's bill for one operation resulted in a $200,000 payment through the IDR process for a self-funded employer. * **Private Equity Firms:** Radiology Partners, TeamHealth, and SCP are named as examples of private equity-owned physician groups heavily utilizing the IDR process. * **Geographic Hotspots:** Texas, Florida, New Jersey, New York, Arizona, Tennessee, and Georgia are identified as states with high concentrations of IDR cases and provider fraud.

Veeva, Disney, and Nvidia Earnings - Your Daily Stock Analysis
InvestTalk
/@InvestTalkPodcast
Sep 3, 2025
This video provides a detailed stock analysis of several companies, including a significant focus on Veeva Systems (VEEV) and Regeneron Pharmaceuticals (REGN), both operating within the life sciences sector. It also delves into Nvidia's earnings, discussing broader AI chip demand and market trends, alongside other companies like Universal Music Group, Disney, Tetra Tech, and Warby Parker. The analysis covers financial performance, growth projections, market valuations, and industry-specific challenges, concluding with a discussion on the increasing speculation in financial markets. Key Takeaways: * **Veeva Systems' Dominance in Life Sciences Cloud:** The video highlights Veeva Systems as a leading cloud solutions provider for the global life sciences industry, enabling pharmaceutical and other companies to adopt modern cloud-based architectures. Its phenomenal growth (20% average over 5 years) and strong net income projections underscore the robust demand for specialized cloud solutions in this regulated sector. * **Accelerated Technology Adoption in Life Sciences:** Veeva's impressive performance signifies a continued and expanding trend of life sciences companies investing in advanced cloud and digital technologies to enhance operations and maintain regulatory compliance * **Massive Projected AI Spending:** Nvidia's earnings report indicates a projected "three to four trillion in AI spending over 5 years," signaling a substantial and sustained investment in AI capabilities across various industries. * **Geopolitical Influences on Tech Supply Chains:** The impact on Nvidia's data center revenue due to restrictions on H20 chip sales to China illustrates how geopolitical factors can influence the availability and adoption of advanced technologies, a consideration for implementing complex AI solutions in regulated environments. * **Divergent Performance of Pharma vs. Pharma Tech:** The contrasting performance between Veeva Systems (strong growth in life sciences tech) and Regeneron Pharmaceuticals (slower growth, underperformance in traditional biotech) underscores the value proposition of technology solutions in helping pharmaceutical companies navigate market volatility and optimize their operations.

Medical Fraud Waste and Abuse Explained
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Sep 1, 2025
This video provides an in-depth explanation of medical fraud, waste, and abuse (FWA), a significant issue estimated by the FBI to account for 3-10% of all healthcare spending. Dr. Eric Bricker, the speaker, breaks down FWA into four distinct categories as defined by the Centers for Medicare and Medicaid Services (CMS): mistakes, waste, abuse, and deliberate fraud, focusing on their impact on employer-sponsored health plans. He emphasizes that while insurance carriers are theoretically responsible for catching FWA, their automated claims processing and historical refusal to allow external audits often lead to substantial financial losses for self-funded employers. The presentation delves into each FWA category with vivid, real-world examples. "Mistakes" are illustrated by a $250,000 infusion medication claim that was double-billed to both a medical plan and a pharmacy benefits manager (PBM) by the same carrier, resulting in a half-million-dollar payout for a quarter-million-dollar service. "Waste" is exemplified by a healthy, normal-risk pregnant woman receiving monthly ultrasounds without clinical indication. "Abuse" is explained through "upcoding," where conditions like pneumonia or urinary tract infections were re-coded as sepsis to secure higher Medicare and commercial plan reimbursements, despite no change in patient management or hospital costs. The most egregious category, "deliberate fraud," is highlighted with two impactful case studies reported by ProPublica. One involves a personal trainer for Southwest Airlines employees who billed over $4 million in physical therapy claims over several years, despite not being a licensed physical therapist. Another details out-of-network physical therapists in New Jersey charging $667 per "medical massage" to a state employee health plan with rich out-of-network benefits, a clear scam. Dr. Bricker points out that carriers often auto-adjudicate claims under $10,000-$15,000 without human review, allowing such fraud to persist, particularly in self-funded plans where the carrier bears no risk. He also addresses the common counter-argument that prior authorizations balance out FWA, illustrating with a diagram how both problems can co-exist and impact different providers, meaning neither should be ignored. The video concludes by offering a concrete solution: independent auditing. Dr. Bricker advocates for employers and benefits consultants to hire separate data analytics firms specifically to review claims for FWA, thereby auditing the carrier's performance. He cites a benefits consultant who reduced health plan costs by 6% for large Fortune 500 companies by strictly addressing FWA. Another example includes an insurance captive that has kept healthcare costs flat for over a decade by employing two outside data analytics vendors to scrutinize carrier data. This "trust but verify" approach, enabled by demanding data access from carriers or switching to those who comply, is presented as a proven method to reclaim significant healthcare spending. Key Takeaways: * **Significant Financial Impact of FWA:** Medical Fraud, Waste, and Abuse (FWA) accounts for an estimated 3-10% of all healthcare spending, representing a substantial financial drain on health plans, including employer-sponsored ones. * **Four Categories of FWA:** CMS categorizes FWA into Mistakes (e.g., double billing), Waste (e.g., unnecessary procedures), Abuse (e.g., upcoding for higher reimbursement), and Deliberate Fraud (e.g., billing for services not rendered or by unqualified personnel). * **Carrier Limitations in FWA Detection:** Traditional insurance carriers often fail to detect FWA due to automated claims adjudication processes for claims under $10,000-$15,000 and a historical reluctance to allow external audits of their FWA departments. * **"Trust But Verify" is Essential:** For self-funded plans, carriers bear no risk for FWA, making it crucial for employers to implement a "trust but verify" strategy rather than solely relying on carrier assurances. * **FWA and Prior Authorization Are Separate Issues:** The existence of egregious prior authorization denials does not negate the need to address FWA; both are distinct problems that require separate solutions and can impact different providers. * **Independent Data Analytics as a Solution:** The most effective strategy to combat FWA is to hire independent, third-party data analytics firms to review claims data and audit the carrier's FWA detection efforts. * **Actionable Steps for Employers:** Employers should demand access to their claims data for independent review. If a carrier refuses, they should consider issuing an RFP (Request for Proposal) to find a new carrier that allows such audits. * **Tangible Cost Savings:** Addressing FWA through independent auditing can lead to significant cost reductions, with one benefits consultant reporting a 6% decrease in health plan costs for large employers. * **Empowering Claims Management:** With independent data analysis, employers can instruct carriers to stop payments on fraudulent claims or withhold future payments to providers who have previously received fraudulent payouts. * **Proven Success Models:** Examples like an insurance captive maintaining flat healthcare costs for over a decade by utilizing multiple outside FWA auditing vendors demonstrate the efficacy of this approach. * **Data Access is Paramount:** The ability to access and analyze comprehensive claims data is fundamental for identifying patterns of FWA that automated carrier systems often miss. **Key Concepts:** * **FWA (Fraud, Waste, and Abuse):** A broad term encompassing intentional deception (fraud), inefficient or unnecessary use of resources (waste), and practices that directly or indirectly result in unnecessary costs (abuse). * **Upcoding:** Billing for a more expensive service or diagnosis than what was actually provided or justified, often to increase reimbursement. * **Auto-adjudication:** The automated processing and payment of claims by an insurance carrier's computer system without human review, typically for claims below a certain monetary threshold. * **Self-funded Plan:** An employer-sponsored health plan where the employer directly pays for employees' healthcare costs rather than paying premiums to an insurance carrier, making them directly responsible for FWA losses. * **Prior Authorization:** A requirement from an insurance plan that a healthcare provider obtain approval before providing a service or prescribing a medication, often used to control costs and ensure medical necessity. * **RFP (Request for Proposal):** A document issued by an organization to solicit bids from potential suppliers for a specific project or service, used here to find carriers willing to allow independent FWA audits. **Examples/Case Studies:** * **Double-billed Infusion Medication:** A $250,000 infusion drug was billed twice (medical and pharmacy claims) by the same carrier/PBM, resulting in a $500,000 payout for a $250,000 service. * **Unnecessary Monthly Ultrasounds:** A healthy, normal-risk pregnant woman received monthly ultrasounds without clinical indication, representing medical waste. * **Sepsis Upcoding:** A significant increase in sepsis diagnoses (from 248,000 to 541,000 cases) was observed after Medicare increased reimbursement, with conditions like pneumonia being re-coded as sepsis to gain higher payments without changes in patient care. * **Southwest Airlines Personal Trainer Fraud:** A personal trainer billed Southwest Airlines' employee health plan over $4 million for physical therapy claims, despite not being a physical therapist. * **New Jersey Out-of-Network Massage Fraud:** Out-of-network physical therapists in New Jersey billed $667 per "medical massage" to a state employee health plan with generous out-of-network benefits, amounting to significant fraud. * **Benefits Consultant Cost Reduction:** A benefits consultant achieved a 6% reduction in health plan costs for Fortune 500 employers by rigorously addressing FWA through independent auditing. * **Insurance Captive Flat Costs:** An insurance captive maintained flat healthcare costs for over 10 years by employing two outside data analytics vendors to audit carrier claims for FWA.

My Take on Veeva's Strong Q2 Financials
Corporate Decoder
/@CorporateDecoder
Aug 30, 2025
This video provides a financial analysis of Veeva Systems' second quarter fiscal 2026 results, highlighting strong performance across key metrics. The speaker details Veeva's revenue growth, profitability, and cash flow, positioning the company as a robust cloud-based software provider for the life sciences industry. The analysis delves into the breakdown of revenue streams, noting significant growth in customer services, and discusses the implications of these numbers for Veeva's market position and operational efficiency. Key Takeaways: * **Veeva's Strong Financial Health:** Veeva Systems reported impressive Q2 fiscal 2026 results, with total revenue increasing 15% year-over-year to $779 million, operating income up 20%, and net income surging 25%, demonstrating robust growth and profitability. * **Customer Services Driving Engagement:** Customer services revenue saw a substantial 21% year-over-year increase, outpacing the 12% growth in subscription revenue. This indicates a deepening relationship with existing clients, suggesting increased adoption of additional support, consulting, and expanded use of Veeva's platforms. * **Operational Efficiency and Leverage:** The faster growth in operating income (20%) and net income (25%) compared to total revenue (15%) signifies that Veeva is effectively managing its costs and achieving operational leverage, translating top-line growth into even stronger bottom-line results. * **Stable and Growing Ecosystem:** Veeva's consistent growth and specialization in the life sciences industry create a stable and expanding ecosystem. Its strong financial trajectory underscores the ongoing demand for specialized software and services within the pharmaceutical and biotech sectors.
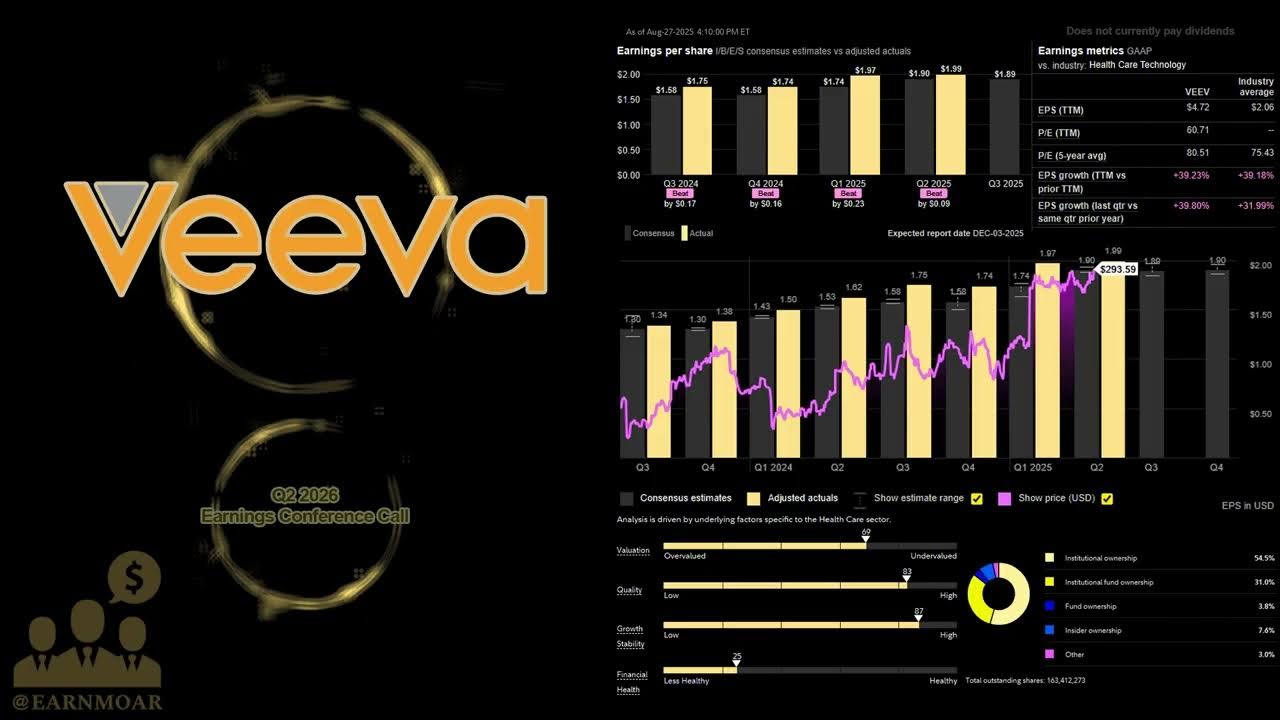
$VEEV Veeva Systems Q2 2026 Earnings Conference Call
EARNMOAR
/@EarnMoar
Aug 27, 2025
This video presents the Veeva Systems fiscal 2026 second quarter earnings conference call, offering a comprehensive update on the company's strategic direction, product advancements, and market performance within the life sciences industry. Key discussions revolved around the transformative potential of Veeva AI, the significant implications of resolving a long-standing lawsuit with IQVIA, and the continued momentum of Vault CRM and other cloud offerings. Management emphasized a vision where industry-specific software, data, and business consulting converge, with AI agents playing a pivotal role in enhancing efficiency and effectiveness across commercial, R&D, and quality operations, all while maintaining a focus on regulatory compliance. Key Takeaways: * **Transformative AI Strategy:** Veeva is making rapid progress on "Veeva AI," deeply embedding agentic AI into its Vault platform to create industry-specific agents. This is expected to be transformative for both Veeva and the life sciences industry, offering billions of dollars in value through increased human efficiency and automation of tasks, though material revenue contribution is not expected until 2027 or later. * **IQVIA Lawsuit Resolution Unlocks Commercial Cloud:** The resolution of the 10-year dispute with IQVIA removes "artificial barriers" that previously hindered Veeva's commercial cloud. This allows for the integration of industry-leading IQVIA data into Veeva Networks and Nitro, making these commercial analytics offerings practical and enabling a more "wholesome" solution that is expected to drive broader adoption of Veeva's commercial suite. * **Strong Vault CRM Momentum:** Veeva reports significant competitive success in the CRM market, with 9 top 20 pharma companies committed to Vault CRM, compared to Salesforce's 3. Two top 20 customers are already live in major markets, demonstrating proven implementation capabilities and a faster time-to-value compared to Salesforce's longer project timelines. * **Agentic AI Interoperability:** Veeva is architecting its AI agents to enable seamless communication and interoperability between agents within the Veeva ecosystem and with agents from other enterprise systems like SAP, Workday, or Microsoft. This "agent-to-agent interoperability" is highlighted as a less brittle and highly beneficial approach for cross-system workflows. * **Business Consulting Critical for AI Adoption:** The company stresses that every AI project is inherently a business consulting project, as it involves redefining workflows and the division of labor between humans and AI agents. This positions Veeva's business consulting services as a crucial component for successful AI implementation and change management within customer organizations. * **Broad-Based Growth Across Clouds:** Beyond commercial, Veeva sees continued strong execution and pipeline build in R&D subscriptions. Quality Cloud is also receiving elevated focus, with expansion into areas like Laboratory Information Management Systems (LIMS) and batch release, indicating its potential as a significant growth driver. * **Crossix Driving Commercial Growth:** Veeva's Crossix product continues to be a meaningful driver of commercial growth, showing strong performance in both its measurement business and the higher-growth audiences segment. This growth is attributed to increasing market share and expanding product footprint, particularly in HCP marketing.

Veeva Systems VEEV Q2 2026 Earnings Call
Fyfull
/@fyfull
Aug 27, 2025
This video provides an in-depth exploration of Veeva Systems' fiscal 2026 second-quarter earnings call, offering significant insights into the company's strategic direction, product advancements, and market performance within the life sciences industry. The call, led by CEO Peter Gastner and other executives, highlights strong financial results and a clear vision for future growth, particularly emphasizing the transformative potential of AI, the strategic impact of resolving a long-standing dispute with IQVIA, and the continued momentum across its core product suites, including CRM, R&D, and Quality Cloud. A central theme of the discussion revolves around "Viva AI" and its integration into the Vault platform. Gastner articulates a vision where AI agents, content, and data will seamlessly interact, creating deep industry-specific applications and significantly expanding Veeva's market opportunity. While acknowledging that material revenue contribution from AI is not expected in the immediate future (2026-2027), the company anticipates creating billions of dollars in value for the industry by enhancing efficiency, automating tasks, and potentially reducing the need for certain types of outsourced labor in areas like safety and clinical operations. The discussion also touches upon the unique "structural advantage" Veeva possesses in AI due to its deeply embedded applications and system-of-record status within customer workflows. Another pivotal development discussed is the resolution of a decade-long lawsuit with IQVIA. This settlement is presented as a major unlock for Veeva's commercial cloud, removing previous restrictions that hindered the full potential of products like Veeva Network and Veeva Nitro, which can now integrate industry-leading IQVIA data. This move is expected to transform Veeva's commercial offerings, making them more comprehensive and practical for customers, fostering greater adoption of the entire commercial suite, and driving stickiness through enhanced business consulting. The call also provides updates on the competitive landscape for Veeva CRM, with the company reporting significant wins against Salesforce and successful go-lives with major pharmaceutical clients, underscoring the growing confidence in Vault CRM. Key Takeaways: * **Transformative AI Vision:** Veeva is making rapid progress on "Viva AI," deeply plumbing it into the Vault platform to create industry-specific agents that interact with content and data. This is expected to be transformative for the industry, generating billions in value by enhancing efficiency and automating tasks. * **Strategic IQVIA Resolution:** The settlement of the 10-year dispute with IQVIA is a major unlock for Veeva's commercial cloud. It removes artificial barriers, allowing products like Veeva Network and Veeva Nitro to integrate IQVIA data, making them more practical and comprehensive. * **Enhanced Commercial Cloud Potential:** The IQVIA resolution and the move to Vault CRM (away from Salesforce reliance) clear the runway for Veeva to significantly expand its commercial revenue by maturing existing applications (Campaign Manager, Service Center, Patient CRM) and driving customer adoption. * **Veeva CRM Dominance:** Veeva continues to gain traction with Vault CRM, reporting nine top 20 pharma companies committed, compared to Salesforce's three. Two top 20 customers are already live in major markets, demonstrating successful implementation and a faster time-to-value compared to Salesforce's longer projected timelines. * **Agentic AI Monetization Strategy:** Monetization of Viva AI will come from selling the platform for customers to create custom agents and, more importantly, from Veeva's industry-specific agents. While not expecting material revenue in 2026-2027, the long-term market size expansion is significant. * **Structural Advantage in AI:** Veeva's deep application integration, where its AI agents are built within existing systems of record (e.g., CRM, TMF), provides a "structural advantage." This allows agents to operate seamlessly within user workflows, adhere to security rules, and perform transactional updates. * **Diverse AI Use Cases:** Agentic AI is expected to impact all areas of life sciences differently. In commercial, it will enable greater productivity; in safety and clinical, it could significantly reduce outsourced labor (e.g., 50% reduction in TMF processing). * **Interoperable Agents:** Veeva is architecting its agents to communicate with agents from other systems (e.g., SAP, Workday, Microsoft Office) using a common protocol (MCP model), which promises less brittle and more seamless cross-system automation. * **Elevated Quality Cloud Focus:** Quality Cloud is receiving increased internal focus, with plans to extend into Laboratory Information Management Systems (LIMS), batch release, and validation management, indicating a significant growth area for Veeva. * **Business Consulting for AI Adoption:** Every AI project is inherently a business consulting project, as it involves changing human-agent workflows. Veeva's business consulting group plays a crucial role in guiding customers through this transformation, which is seen as a major structural advantage for AI adoption. * **Crossix as a Growth Driver:** Crossix, Veeva's commercial data and analytics product, continues to show strong momentum, driven by both market share gains and product footprint expansion (e.g., in audiences and HCP marketing). * **Migration Momentum for Vault CRM:** While some customers are hesitant to migrate from legacy Veeva CRM, the "pull of AI" and the natural progression of time are expected to accelerate migrations, with the bulk anticipated in 2026 and 2027. * **Measured AI Approach with Large Goals:** Veeva is taking a deliberate, platform-first approach to AI, but with very aggressive goals, such as potentially reducing outsourced labor in TMF by 50% across the industry. Partners are expected to play a role in developing agents and interoperating with Veeva's solutions. **Key Concepts:** * **Veeva AI / Agentic AI:** Veeva's proprietary artificial intelligence initiatives focused on creating industry-specific intelligent agents that automate tasks and enhance productivity within life sciences workflows. * **Vault Platform:** Veeva's foundational cloud platform that supports its suite of applications, integrating content, data, and now AI agents. * **Vault CRM:** Veeva's next-generation customer relationship management platform built on the Vault platform, designed specifically for the pharmaceutical industry. * **Veeva Network & Veeva Nitro:** Commercial data and analytics products that, following the IQVIA resolution, can now integrate industry-leading IQVIA data, enhancing their utility and effectiveness. * **Quality Cloud:** Veeva's suite of applications for managing quality processes, with an elevated focus on expanding into areas like LIMS (Laboratory Information Management Systems) and manufacturing. * **Crossix:** Veeva's commercial data and analytics business, focusing on measuring and optimizing marketing to both consumers (patients) and healthcare providers (HCPs). * **TMF (Trial Master File):** A critical collection of documents for clinical trials, an area identified for significant AI-driven automation. * **MCP Model (Context Protocol):** A common protocol enabling seamless agent-to-agent interoperability across different systems and vendors. * **Structural Advantage:** Veeva's inherent competitive edge in AI adoption due to its deep integration into customer workflows and its applications serving as systems of record. **Examples/Case Studies:** * **Vault CRM Go-Lives:** Two top 20 pharmaceutical companies successfully went live with Vault CRM in major markets within two years of commitment. * **TMF Processing Automation:** Agentic AI is envisioned to automate tasks such as document filing, verifying documentation completeness against protocols, and identifying illegible documents within the clinical trial master file. * **Commercial Content Workflow:** An early adopter AI project in commercial content involved intricate workflows between medical, regulatory, legal, different brands, and countries, demonstrating how AI and business consulting can transform complex processes. * **IQVIA Data Integration:** The resolution allows Veeva Network and Nitro to integrate IQVIA data, which was previously a "hole in our boat," making these products more viable and powerful for commercial analytics.

The Tokio-Marine HCC 2025 Stop-Loss Report: 5 Key Takeaways
Self-Funded
@SelfFunded
Aug 15, 2025
This video provides an in-depth exploration of the Tokio Marine HCC 2025 Stop-Loss Report, breaking down five key takeaways that illuminate the current hardening market for stop-loss insurance. The speaker, a self-proclaimed "stop-loss geek," aims to equip listeners with critical knowledge to navigate projected medical cost increases of 9-10% in 2025 and increasingly challenging renewals. The analysis delves into the specific trends driving these shifts, offering a granular look at the frequency and severity of large claims, the surprising cost implications of COBRA claimants, the prevalence of policy "lasers," and the crucial concept of loss ratio maturity. The presentation systematically unpacks the report's findings, starting with diagnostic categories, differentiating between those most frequently leading to large claims (e.g., neoplasms/cancer, cardiovascular diseases) and those driving the highest severity costs (e.g., transplants, congenital anomalies, blood diseases like hemophilia). It then transitions to a striking analysis of large claims over time, revealing a dramatic increase in the frequency of multi-million dollar claims, which have necessitated a redefinition of what constitutes a "catastrophic" claim. The speaker attributes some of these trends to the impact of the Affordable Care Act (ACA), particularly the removal of lifetime and annual maximums, and the rise of expensive treatments like gene therapies. Further insights are provided on the financial burden posed by COBRA claimants, who are shown to be significantly more likely to incur large claims than active employees, prompting a discussion on potential solutions for employers. The video also clarifies the concept of loss ratio maturity, explaining how a policy's loss ratio at different points in its term can predict its final outcome, which is highly relevant for renewal negotiations. Finally, the analysis covers the frequency of "lasers" (specific deductibles for named individuals) on stop-loss policies, comparing market averages with Tokio Marine's own practices and discussing the influence of "no new laser" provisions and captive insurance models. The overarching message is that the market is experiencing a significant rebound and hardening, partly due to underwriters misinterpreting the post-COVID cost plateau as normal rather than a unique event, leading to artificially suppressed rates that are now correcting sharply. Key Takeaways: * **Hardening Stop-Loss Market:** The stop-loss market is experiencing significant hardening, with renewals becoming tougher due to projected medical cost increases of 9-10% in 2025, a trend expected to continue for several years. * **Dramatic Increase in Large Claims:** Claims exceeding $2 million are now 1,250% more frequent than in 2013, while $500,000 claims are up 250%. This necessitates a redefinition of "catastrophic" claims, with $1 million no longer considered exceptionally large and $2 million becoming the new benchmark. * **Key Diagnostic Cost Drivers (Frequency):** Neoplasms and cancer consistently rank as the most frequent large claim categories, followed by cardiovascular, musculoskeletal, and digestive diseases. Nervous system diseases (e.g., Multiple Sclerosis, with expensive medications) have recently moved into the top five. * **Key Diagnostic Cost Drivers (Severity):** Transplants remain the most expensive category, with congenital anomalies (e.g., malformations, premature babies in NICU) and blood diseases (e.g., hemophilia, often requiring costly gene therapies or ongoing medication) also being major drivers of severe costs. * **Impact of Medical Advances:** Advances in medical technology, particularly gene therapies, are significant contributors to the rising cost and duration of large claims, as they often involve ongoing, expensive treatments rather than cures. * **High Cost of COBRA Claimants:** Individuals electing COBRA are substantially more expensive on average than active employees. They are 13.5 times more likely to have a $200,000 claim, 14.1 times more likely for a $500,000 claim, and 15.5 times more likely for a $1 million claim, highlighting a significant risk for plan sponsors. * **Employer Solutions for COBRA:** Employers should consider offering solutions or guidance to COBRA-eligible members to explore open market options. This can help mitigate the significant financial risk associated with retaining high-cost claimants on the employer's plan. * **Loss Ratio Maturity for Renewals:** Understanding loss ratio maturity is crucial for renewals. The further into a policy period (e.g., 9 months), the more predictable the final loss ratio becomes, allowing stop-loss carriers to price renewals more accurately and potentially less conservatively. * **Laser Frequency Trends:** On average, about one in four policies in the market has a "laser" (a specific deductible for a named individual), with an average of one laser per policy. However, carriers like Tokio Marine HCC demonstrate significantly lower laser frequency, suggesting better data, larger case sizes, or higher risk tolerance. * **Influence of "No New Laser" Provisions:** The growth of "no new laser" provisions and captive insurance models is likely contributing to a slight decrease in the overall number of policies that have lasers placed on them. * **Post-COVID Rebound Effect:** The current market hardening is exacerbated by stop-loss underwriters having viewed the cost plateau in 2021-2022 (due to COVID-related deferral of care) as normal credible experience rather than a unique event. This artificially suppressed rates, leading to a more severe "boomerang" effect now. * **Contributing Factors to Rising Costs:** Beyond medical inflation and large claims, other factors include supply and demand dynamics, lower Medicare reimbursements shifting costs to the commercial sector, and the post-COVID rebound in healthcare utilization. Key Concepts: * **Stop-Loss:** Insurance that protects self-funded employers from catastrophic claims. * **Laser:** A specific, higher deductible applied to one or more named individuals on a stop-loss policy, carving out their exposure. * **Loss Ratio Maturity:** The concept that a policy's loss ratio at different points in its term can predict its final loss ratio, with predictability increasing as the policy matures. * **Medical Inflation/Trend:** The rate at which healthcare costs are increasing over time. * **Leverage Trend:** The amplified impact of underlying medical trend on stop-loss costs, as stop-loss covers only the portion of claims above a certain deductible. * **ACA (Affordable Care Act):** Legislation that impacted healthcare coverage, including medical loss ratio (MLR) requirements and the removal of lifetime and annual maximums, contributing to changes in large claim frequency. Tools/Resources Mentioned: * Tokio Marine HCC 2025 Stop-Loss Report (linked in the video description) * Paro (speaker's partner)

What An Intentional Company Culture Looks Like | with Rob Gelb
Self-Funded
@SelfFunded
Aug 1, 2025
This video provides an in-depth exploration of Vālenz Health®'s intentional company culture, as presented by CEO Rob Gelb during an office tour. The primary purpose of the discussion is to illustrate how a deeply ingrained culture, built on specific tenets and philosophies, drives innovation and success within a healthcare company dedicated to making healthcare "smarter, better, and faster." Gelb elaborates on the origins of the company's name, its evolving mission, and the core values that shape its operations, partnerships, and employee experience. The discussion delves into several key themes, beginning with the etymology of "Veilance" (Latin for strong, vigorous, and healthy) and how this concept permeates their brand promise to build strong partnerships and empower individuals in their healthcare journeys. Gelb explains how their mission statement, initially focused on optimizing cost, quality, and utilization, was refined to emphasize "high-value healthcare" and patient protection after a strategic acquisition. A significant portion of the video is dedicated to Vālenz Health®'s unique cultural tenets, such as encouraging "spilled milk" – a metaphor for celebrating learning from failures – and adopting a "yes, and" mindset, borrowed from improvisational comedy, to foster collaborative idea generation and trust among team members and partners. The progression of ideas moves from foundational identity to operational philosophy and leadership principles. Gelb highlights how language is intentionally used throughout their office design to communicate core values, such as breaking down "challenge" into "look, listen, enable" to drive change, and balancing "aspirational" dreams with "perspirational" execution. The conversation also covers the importance of fearlessness, embracing criticism as a gift, and building "player-led" teams where accountability is shared. The video culminates with a powerful affirmation of their innovative culture, evidenced by customer feedback collected in a word cloud where "innovative" was overwhelmingly the largest and most frequent descriptor, reinforcing that their cultural practices directly translate into how they are perceived by their clients. Key Takeaways: * **Intentional Culture as a Strategic Asset:** Vālenz Health® demonstrates how a meticulously crafted company culture, from its name's meaning to its office aesthetics, serves as a foundational pillar for its business strategy and market differentiation. This intentionality helps align employees, partners, and customers with the company's core values. * **Evolving Mission for Refined Focus:** The company's mission statement, initially broad, was strategically refined to "optimized utilization, high-value healthcare" and "protecting patients and helping employers." This highlights the importance of adapting a mission as the company grows and gains deeper market understanding, ensuring it remains relevant and impactful. * **"Smarter, Better, Faster Healthcare" as a Brand Promise:** Vālenz Health®'s brand promise emphasizes efficiency and effectiveness in healthcare delivery, with cost optimization being an implicit outcome rather than the sole focus. This positions them as a value-driven partner focused on holistic improvements. * **Embracing "Spilled Milk" for Learning and Growth:** A core cultural tenet is the encouragement and celebration of "spilled milk," meaning learning from failures. This fosters psychological safety, allowing employees at all levels to experiment, take risks, and grow without fear of punitive consequences. * **The "Yes, And" Mindset for Collaboration:** Inspired by improvisational comedy, the "yes, and" principle promotes additive thinking and collaborative storytelling within the company and with partners. This encourages building upon ideas rather than shutting them down, fostering innovation and stronger relationships. * **Language as a Cultural Driver:** Rob Gelb emphasizes the deliberate use of language and wordplay to instill company values, such as dissecting "challenge" to reveal "change" (Look, Listen, Enable) or balancing "aspirational" dreams with "perspirational" execution. This shows how words can be powerful tools for shaping mindset and behavior. * **Fearlessness and Limitlessness in Innovation:** The company promotes a culture of being "limitless" and "fearless," encouraging employees to dream big, be unafraid to fail, speak unpopular truths, and view criticism as a valuable gift. This mindset is crucial for continuous innovation and breaking new ground. * **Player-Led Teams for Enhanced Accountability:** Vālenz Health® aspires to be a "player-led" organization, where every team member understands their role, knows how to execute for success, and holds peers accountable. This decentralizes leadership and empowers individuals, leading to higher performance and ownership. * **Consistency Over Occasional Greatness:** The philosophy of striving to be "consistently good" rather than "occasionally great" underscores the importance of maintaining high standards and reducing performance variability. This leads to more reliable outcomes and builds trust with partners and customers. * **Customer-Validated Innovation as an Affirmation:** The video highlights how customer feedback, specifically a word cloud overwhelmingly featuring "innovative," serves as a powerful affirmation of their culture. This external validation reinforces that their internal practices of embracing failure and continuous learning translate into perceived innovation by their market. * **Focus on Unmet and Underserved Needs:** Vālenz Health® approaches potential clients not with a sales pitch, but with an invitation to discuss their "unmet and underserved needs" in healthcare. This problem-solving orientation positions them as a caring partner genuinely interested in finding solutions. Key Concepts: * **Vālenz (Veilance):** Latin for strong, vigorous, and healthy, reflecting the company's aspiration for its business, partnerships, and members. * **Spilled Milk:** A metaphor for learning from failures, encouraged as a vital part of growth and innovation. * **Yes, And:** A principle from improvisational comedy adopted to foster collaborative idea generation and build trust by accepting and expanding on others' contributions. * **Challenge = Change:** An internal philosophy where "challenge" is broken down into "Look, Listen, Enable" to facilitate meaningful transformation. * **Aspirational & Perspirational:** The balance between dreaming big (aspirational) and executing diligently (perspirational) to achieve goals. * **Player-Led Organization:** A team structure where individuals are empowered to understand their roles, execute, and hold each other accountable, rather than relying solely on top-down leadership. * **Unmet/Underserved Needs:** The core of Vālenz Health®'s problem-solving approach, focusing on identifying and addressing gaps in the healthcare industry.

Veeva CTV Tutorial
LXReady
/@LXReadylearningexperience
Aug 1, 2025
This video provides a comprehensive tutorial on Veeva's Clinical Trial Viewer (CTV), a free online tool designed to help users find open clinical trials based on various criteria. The walkthrough covers navigating the CTV homepage, understanding the difference between searching by site and by trial, and utilizing advanced search functionalities. It details how to refine searches using filters such as condition, location, trial status (including active, enrolling, completed, and various inactive states), trial type (interventional, observational, expanded access), funder, placebo use, phase, sponsor, site, distance, demographics (age, sex), and trial dates. The tutorial also highlights features for saving and sharing customized search results, emphasizing its utility for researchers, coordinators, and healthcare professionals in streamlining patient recruitment and exploring ongoing studies. Key Takeaways: * **Veeva Ecosystem Integration:** The video demonstrates a key tool within the broader Veeva ecosystem. * **Clinical Operations and Data Management:** CTV directly addresses the core functions of clinical operations and clinical data management by providing a structured way to access and filter clinical trial information * **Granular Trial Search Capabilities:** The platform offers extensive and granular filtering options for clinical trials, including status, phase, sponsor, and demographics, which can be critical for patient recruitment, competitive intelligence, and strategic planning within pharmaceutical and biotech companies. * **Strategic Foresight with Inactive Trials:** The ability to search for and save inactive trials offers a strategic advantage, allowing companies to monitor potential future studies and prepare for enrollment as soon as they become active again.

Deep Dive: Why Veeva Systems Dominates Life Sciences Software (VEEV)
Fundamental Deep Dive
/@fundamental_deep_dive
Jul 31, 2025
This video provides a comprehensive deep dive into Veeva Systems, a dominant cloud software and platform provider exclusively for the life sciences industry. It explores Veeva's specialized business model, which focuses on solving complex challenges in clinical trials, regulatory compliance, and tailored CRM/sales for big pharma, biotech, and medical device companies. The discussion covers Veeva's product ecosystem, built on its proprietary Vault platform, encompassing both Development Cloud (R&D, clinical trial management, regulatory submissions) and Commercial Cloud (sales, marketing automation, data products). A significant theme is Veeva's strategic migration of its CRM from Salesforce technology to its own Vault platform, aiming for increased margins and tighter integration. The video also highlights Veeva's major AI initiatives, embedding AI into its applications for automation and productivity, particularly in areas like Medical Legal Regulatory (MLR) review. Financial performance, competitive landscape, and Veeva's unique Public Benefit Corporation (PBC) status, which fosters deep customer trust, are also key topics, reinforcing the value of deep vertical specialization and long-term commitment in a highly regulated industry. Key Takeaways: * **Validation of Deep Vertical Specialization:** Veeva's unparalleled success underscores the immense value of a laser-like focus on a single, complex industry like life sciences. * **Strategic Importance of Veeva CRM & Platform Migration:** The ongoing, monumental migration of Veeva CRM to the Vault platform presents both challenges and opportunities.ai, this reinforces the critical need for expert Veeva CRM consulting services to help clients navigate these transitions, maximize their CRM investment, and leverage the integrated R&D and commercial capabilities. This highlights practical applications for intelligent automation in regulated environments, emphasizing productivity gains and compliance streamlining. * **Regulatory Compliance as a Foundational Competitive Advantage:** The video consistently emphasizes how Veeva bakes regulatory rules, data integrity, and compliance into its software, making it indispensable. This reinforces that deep regulatory knowledge and the ability to build compliant solutions are not just features but fundamental competitive differentiators in the pharmaceutical and life sciences sectors. * **Untapped Market Potential and Growth Opportunities:** Despite Veeva's dominance, its estimated market penetration is still relatively low within a $20 billion total addressable market in life sciences.ai to expand its client base. * **Trust as a Strategic Differentiator:** Veeva's commitment as a Public Benefit Corporation (PBC) and its emphasis on creating value for customers beyond short-term profit builds immense trust. This suggests that demonstrating long-term commitment, ethical practices, and a deep understanding of stakeholder needs can be a powerful, hard-to-copy competitive strategy in the sensitive life sciences industry.

Veeva eConsent training
vcuresearch
/@vcuresearch
Jul 30, 2025
This video provides an in-depth training session on the functionality and implementation of Veeva eConsent, a new electronic consenting platform integrated within Veeva SiteVault. Lauren Wallace, Director of Clinical Research Regulatory Affairs, guides viewers through the step-by-step process of setting up, configuring, and executing participant consents using this system. The primary goal is to offer an alternative to traditional paper consents, DocuSign, or RedCap, emphasizing regulatory compliance and operational efficiency within clinical research settings. The session covers both in-person and remote consenting options, highlighting how the platform streamlines workflows and ensures adherence to FDA regulations. The training begins with the foundational steps of enabling eConsent within Veeva SiteVault for a specific study, a process that requires site admin privileges. It then delves into the critical task of building and configuring consent forms, demonstrating how to upload IRB-stamped PDF documents and add interactive fields such as radio buttons, checkboxes, and text entry boxes for participant responses and initials. A key focus is placed on mapping signature blocks for various roles, including participants, site members, witnesses, and other potential signatories like guardians or translators. The video also explains how to preview the configured consent form from a participant's perspective before approving it for live use. The demonstration proceeds to the actual consenting process, illustrating an in-person scenario using a site device. It meticulously details the participant's journey, from reviewing each page of the consent form and interacting with the configured fields to digitally signing the document. The workflow for additional signatories, such as a witness, is also covered, showcasing how tasks are routed within SiteVault. A significant advantage highlighted is the automatic filing of completed, signed consent forms directly into the study binder within SiteVault, reducing manual administrative burdens. The session concludes by addressing common questions, discussing the platform's current limitations, and providing resources for further support, reinforcing the importance of obtaining IRB approval before deploying Veeva eConsent for any study. Key Takeaways: * **Veeva eConsent as a Compliant Alternative:** Veeva eConsent is presented as a fully compliant electronic consenting option for clinical research, meeting FDA regulations and offering a Part 11 compliant alternative to paper, DocuSign, or RedCap (which is noted as not Part 11 compliant). * **Mandatory IRB Approval:** Before using Veeva eConsent for any new or existing study, it is a strict requirement to submit an amendment and obtain approval from the Institutional Review Board (IRB) of record, similar to other electronic consenting methods. * **Seamless SiteVault Integration:** A primary benefit of Veeva eConsent is its direct integration with Veeva SiteVault, which automatically files the fully executed consent form into the study's site binder, eliminating manual document management steps. * **Flexible Consent Configuration:** The platform allows for the upload of IRB-stamped PDF consent forms, enabling users to add various interactive fields such as radio buttons (for single-choice selections), checkboxes (for multiple selections), and text entry boxes (for initials or specific inputs). * **Multiple Signatory Roles:** Veeva eConsent supports configuring signature blocks for a wide range of roles, including participants, site members, witnesses, guardians, translators, and legally authorized representatives (LARs), with the flexibility to select applicable signatories per participant. * **Diverse Consenting Methods:** Users can conduct in-person consents using either a VCU-provided device (e.g., computer, tablet) or the participant's own device via a QR code. A fully remote consent option is also available, requiring the participant's email address. * **Guided Participant Experience:** The system ensures participants review every page of the consent form by requiring them to scroll through and interact with fields, preventing quick skips. Checkboxes appear on the side of each page to confirm review. * **Automated Workflow for Staff:** Once participant and witness signatures are complete, the system automatically routes the consent to the designated consenting staff member for review and final signature, with options to further route to the Principal Investigator (PI) if needed. * **Digital Copy for Participants:** Participants can receive a digital copy of their signed consent form through the free My Veeva app (available on Apple, Android, and web), which requires a one-time activation code per study. Alternatively, a PDF can be downloaded, printed, or emailed to the participant. * **Current System Limitations:** As of the training, Veeva eConsent has some limitations, such as only allowing one signature per individual role (e.g., two participant signatures are not supported on the same form) and the site staff/PI signatures appearing on a separate signature page rather than directly on the physical consent document. * **Support for Pediatric Assent:** Veeva eConsent can be used for pediatric assent, allowing for two-parent signatures and consenting participants under 18, provided the IRB approves it as a valid method based on age. * **Importance of Quality Checks:** For non-required fields (e.g., optional research consent), the system does not flag if an option isn't selected, emphasizing the need for manual quality checks by staff. * **Participant ID Flexibility:** While a participant ID is required to initiate the process, it can be changed later if the initial entry was a placeholder or incorrect. Tools/Resources Mentioned: * **Veeva eConsent:** The electronic consenting platform. * **Veeva SiteVault:** The document management system within which eConsent is integrated. * **My Veeva App:** A free application for participants to access their signed digital consent forms. * **DocuSign:** An alternative electronic signature platform, noted as Part 11 compliant. * **RedCap:** An alternative electronic data capture and survey tool, noted as not Part 11 compliant. * **OV Puriz Veeva Page:** An internal resource providing an eConsent SOP. * **Veeva eConsent Help Page:** External resource offering video demonstrations and how-to instructions. Key Concepts: * **eConsent:** Electronic informed consent, a digital method for obtaining participant consent in clinical research. * **IRB (Institutional Review Board):** An administrative body established to protect the rights and welfare of human research subjects. * **FDA Regulations:** Rules and guidelines set forth by the U.S. Food and Drug Administration, particularly relevant for clinical trials and electronic records (e.g., 21 CFR Part 11). * **21 CFR Part 11:** Regulations concerning electronic records and electronic signatures, ensuring their trustworthiness, reliability, and equivalence to paper records. * **HIPAA Authorization:** A document that permits the use or disclosure of an individual's protected health information (PHI) for research purposes. * **Assent:** The agreement of a minor to participate in research, typically in addition to parental permission. * **L (Legally Authorized Representative):** An individual authorized under applicable law to consent on behalf of a prospective subject to the subject's participation in the procedure(s) involved in the research.