veeva rim
24 videos

This OVERSOLD growth stock could have HUGE revenue in 2021 (30% upside potential)
Raylin Records
/@RaylinRecords
Mar 20, 2021
This.ai, and delves into the digital transformation occurring within the pharmaceutical and life sciences industries, particularly concerning clinical trials and operational efficiency. This video explores Veeva ($VEEV) as a leading cloud solution provider driving digitalization in the healthcare sector, specifically within the pharmaceutical and life sciences industries. The speaker discusses Veeva's financial performance and significant growth, highlighting its role in accelerating the shift towards digital, paperless, and patient-centric clinical trials. A major focus is placed on Veeva Vault Clinical applications and the groundbreaking Veeva e-consent, which enables electronic informed consent for clinical studies, streamlining processes and facilitating decentralized trials. The video emphasizes the vast market opportunity for digital healthcare solutions and the increasing adoption of cloud-based services accelerated by recent global events. Key Takeaways: * **Veeva's Central Role in Digital Healthcare:** Veeva is presented as a crucial cloud solution for healthcare, offering business consulting and training to major pharmaceutical clients like Merck, Moderna, and Eli Lilly, underscoring its deep integration into the industry. * **Pioneering Digital Consent and Decentralized Trials:** The introduction of Veeva e-consent marks a significant milestone, enabling the first fully digital, electronic informed consent in clinical trials. This innovation facilitates paperless, patient-centric studies and supports a "work from home study" model, allowing remote patient participation and real-time visibility for sponsors. * **Streamlining Clinical Operations:** Veeva Vault Clinical applications are highlighted for their ability to streamline global trial processes for Contract Research Organizations (CROs), enhancing efficiency, reducing administrative burden, and accelerating study execution. * **Massive Untapped Market Potential:** The digital healthcare community represents a substantial and growing total addressable market (TAM), estimated at over $12 billion. Veeva is positioned to capture a significant portion of this, with projections for substantial revenue growth across its diverse cloud services, including data cloud, patient data & analytics, and commercial cloud. * **Accelerated Digital Adoption Post-Pandemic:** The video notes that the pandemic has significantly accelerated the industry's move towards digital solutions, making cloud-based services essential for maintaining study timelines and improving overall trial execution. * **Focus on Efficiency and Patient Experience:** The core benefits of Veeva's innovations are increased efficiency, faster processes, reduced administrative tasks, and an improved patient experience by removing geographical and paper-based barriers in clinical research.

Ending the paper trail with REDCap: optimized workflows for screening, consenting, and compensating
MGH Martinos Center
/@MGHMartinosCenter
Apr 11, 2020
This video explores the successful digital transformation of administrative workflows in clinical research at the MGH Martinos Center, specifically focusing on replacing paper-based processes with REDCap. Olivia Rowe, a Clinical Research Coordinator, details how her team optimized subject screening, consenting, and compensation by leveraging REDCap's capabilities. The presentation highlights the numerous inefficiencies and compliance risks associated with paper forms, such as space consumption, outdated information, HIPAA vulnerabilities, and lack of flexibility. The implemented REDCap solution introduces digital safety screening, dynamic branching logic for tailored questionnaires (e.g., pregnancy screening), real-time eligibility feedback for participants, digital sign-offs for MRI technicians, and an integrated subject payment system. This initiative aims to alleviate administrative burdens, enhance data accuracy, and significantly improve regulatory compliance through robust audit trails and streamlined processes. Key Takeaways: * **Digital Transformation in Clinical Research:** The video directly addresses the administrative burden and compliance risks of paper-based workflows in clinical research, demonstrating a clear need for digital solutions to optimize operations within the life sciences sector. * **Workflow Optimization and Automation:** REDCap is effectively utilized to streamline critical processes like subject screening, consent, and compensation, showcasing the value of automating traditionally manual tasks through digital forms with features like branching logic and real-time feedback. * **Enhanced Regulatory Compliance:** Moving to digital platforms like REDCap significantly improves HIPAA compliance, provides thorough audit trails, and simplifies IRB approval processes through templated amendments, aligning with the strict regulatory requirements (e.g.ai helps clients navigate. * **Improved Participant Experience and Data Quality:** Digital forms empower participants to provide documentation, receive immediate eligibility feedback, and ensure more accurate and relevant data collection, reducing administrative overhead for research staff and improving overall data integrity. * **Interoperability and Customization Potential:** The discussion touches upon the ability to integrate with EMRs (like Epic) and the customizability of REDCap forms, highlighting the flexibility required for diverse research needs and the potential for broader data engineering initiatives. * **Scalability through Data Dictionaries:** The concept of sharing forms via a "data dictionary" allows for efficient replication and customization of standardized questionnaires across different labs, reducing redundant effort in setting up digital tools and promoting best practices.

End to End Regulatory Information Management Case Study of a Veeva RIM Implementation
Astrix On Demand Webinars for Life Sciences
/@astrixlifescience
Apr 19, 2023
This. The discussion covers critical aspects like harmonizing global regulatory processes, managing data migration and integration from legacy systems, ensuring compliance, and navigating large-scale enterprise software adoption within the life sciences industry. This video explores an end-to-end Veeva Regulatory Information Management (RIM) system implementation for a top 10 pharmaceutical company, transitioning from disparate legacy systems to a globally consistent solution. The speakers discuss the complexities of harmonizing processes and terminology across global teams managing regulatory, CMC, and safety submissions. The case study highlights a multi-year journey involving current state assessment, future state design, detailed requirements gathering, configuration, and phased rollout strategies. A key theme is the collaborative approach between the client, Astrix (the consulting firm), and Veeva, emphasizing strong program management, communication, and meticulous planning for process and data readiness, as well as user adoption in a highly regulated environment. Key Takeaways: * **Complex Global Harmonization:** Large pharmaceutical companies face significant challenges in harmonizing regulatory processes and terminology across global teams due to reliance on disparate legacy tools (spreadsheets, SharePoint, bespoke systems). * **Structured Multi-Workstream Approach:** Successful large-scale implementations require a highly structured approach with dedicated workstreams for different functional areas (e.g., CMC, Safety, Regulatory, Authoring, Archive, Labeling), ensuring focused effort and expertise. * **Criticality of Process & Data Readiness:** Meticulous assessment of current processes, definition of harmonized future states, alignment on terminology, and comprehensive data mapping, migration, and integration planning are fundamental to a successful RIM system rollout. * **Vendor Collaboration is Key:** Direct and continuous collaboration with the software vendor (Veeva) is crucial for understanding system capabilities, best practices, configuration options, and leveraging product roadmaps for future enhancements. * **Comprehensive Change Management & Adoption:** Effective user adoption plans, including stakeholder analysis, continuous communication (newsletters, intranet), and tailored training, are essential to manage the learning curve and ensure business continuity during phased rollouts. * **Navigating Implementation Hurdles:** Common challenges include managing team member bandwidth, coordinating global time zones, addressing workstream dependencies, and adapting to system changes while maintaining ongoing critical operations and submission deadlines. * **Phased Rollout Strategy:** An iterative, phased rollout, starting with core capabilities and gradually expanding scope based on system readiness, legacy constraints, and business continuity, is an effective strategy for managing large-scale enterprise software implementations.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Oct 1, 2021
This video directly addresses Regulatory Information Management Systems (RIMS) within the pharmaceutical and life sciences industries, a core area for companies seeking to optimize operations and maintain regulatory compliance. **Summary:** The "RegTalks" video, featuring Lidia Canovas from Asphalion and Frank Dickert from EXTEDO, provides an in-depth exploration of Regulatory Information Management Systems (RIMS) and their increasing importance in the pharmaceutical and life sciences sectors. The speakers define RIMS as comprehensive solutions for master product data management, regulatory activity tracking, and integrated document management, serving as a crucial "single source of truth." A central theme is the significant industry transition from xEVMPD to the complex ISO IDMP standard, which necessitates robust RIMS to handle increased data transmission and regulatory compliance. The discussion highlights how RIMS enhance operational efficiency by replacing fragmented manual systems, improve impact assessment capabilities, and are applicable across various regulated fields beyond human medicines, including animal health and medical devices. Furthermore, the video emphasizes that RIMS benefit a wide array of departments beyond regulatory affairs, such as quality, clinical, manufacturing, and supply chain, and stresses the indispensable role of experienced implementation partners in successfully deploying these complex systems. **Key Takeaways:** * **RIMS as a Foundational Compliance System:** Regulatory Information Management Systems (RIMS) are critical enterprise solutions for the life sciences, providing a "single source of truth" for master product data, regulatory activities, and integrated document management, essential for ensuring regulatory compliance and operational efficiency. * **IDMP Transition Drives RIMS Adoption:** The impending transition to the ISO IDMP standard is a major catalyst for RIMS implementation, as it significantly increases data complexity and submission requirements, necessitating sophisticated systems to manage and submit data effectively, often integrated with eCTD submissions. * **Broad Organizational Impact:** RIMS are not exclusive to regulatory affairs but serve a wide range of departments across a life sciences company, including quality, clinical, manufacturing, marketing, and supply chain, by providing centralized access to regulatory information, thereby improving cross-functional decision-making and impact assessment. * **Beyond Human Medicines:** The utility of RIMS extends beyond human pharmaceuticals to other regulated sectors such as animal health, consumer health, and medical devices (e.g., for IVDR/MDR compliance), indicating a broader market need for robust regulatory data management solutions. * **Criticality of Implementation Expertise:** Successful RIMS deployment, especially in the context of IDMP compliance, requires knowledgeable external implementation partners who can provide technical support, guide necessary process changes, and leverage deep industry experience to ensure optimal system configuration and user adoption.

What is a Clinical Trial Manager (CTM) | Salary, Degree Requirements & More
kyyah abdul
/@kyyahabdul
Aug 30, 2022
This video provides a comprehensive overview of the Clinical Trial Manager (CTM) role, detailing their responsibilities, necessary educational background, and salary expectations. The speaker emphasizes that CTMs are crucial for establishing productive vendor relationships, ensuring strict clinical trial compliance with federal and global regulations, and improving the overall efficiency and quality of clinical trial activities. Key duties include overseeing Contract Research Organizations (CROs), managing investigational product sites, participating in study startup, reviewing critical study documents, resolving operational issues, and meticulously preparing and adhering to budgets and timelines. The CTM role is described as a blend of business acumen and scientific understanding, often requiring prior experience as a Clinical Research Associate (CRA). Key Takeaways: * **Central Role in Clinical Operations:** The CTM is a pivotal figure in pharmaceutical and biotech companies, directly responsible for the oversight and successful execution of clinical studies, bridging scientific objectives with operational realities. * **Critical for Regulatory Compliance:** A primary responsibility of the CTM is to ensure clinical trial compliance with federal and global regulations, highlighting the high-stakes environment where regulatory adherence is paramount. * **Complex Vendor Management:** CTMs are deeply involved in managing relationships with various vendors, particularly CROs, requiring strong negotiation, oversight, and problem-solving skills to maintain study timelines and budgets. * **Operational Efficiency and Problem Solving:** The role demands proactive identification and swift resolution of operational challenges to enhance the efficiency, effectiveness, and quality of clinical trial activities, directly impacting study success. * **Data-Driven Decision Making:** The CTM's need to manage study progress, budgets, and timelines implies a reliance on robust data and analytics for informed decision-making and issue resolution.

Aquila University The eCTD Publishing Process
Aquila Solutions
/@aquilasolutions6783
Jan 17, 2020
This video explores the intricate and often underestimated eCTD (Electronic Common Technical Document) publishing process, which is critical for regulatory submissions like NDAs to agencies such as the FDA. The speaker highlights the common misconception among sponsors regarding the time and resources required to convert years of clinical data into a compliant and reviewable eCTD, emphasizing that inadequate planning significantly increases project risk. The discussion details the essential components for eCTD creation, including specialized publishing systems, advanced PDF tools, eCTD validators, and a dedicated team of experienced regulatory operations publishers. The process is broken down into four major activities: lifecycle planning, document remediation, quality control, and project management, with remediation consuming the vast majority of the effort. Key Takeaways: * **Underestimated Complexity:** eCTD publishing is a highly complex, resource-intensive process, with sponsors frequently underestimating the 2,000 man-hours and minimum four months required for a typical 505 B1 NDA (100,000+ pages, 300+ documents). * **Critical Resource Requirements:** Successful eCTD publishing necessitates a robust technology stack (publishing system, advanced PDF tools, dual eCTD validators) and a skilled team comprising experienced manager-level and associate-level regulatory publishers. * **Remediation is King:** Document remediation, involving cleaning, formatting, bookmarking, and intricate linking of individual PDFs, accounts for approximately 70% of the total project time, underscoring its manual and meticulous nature. * **QC is Non-Negotiable:** Cutting corners on the 15% allocated for quality control introduces substantial risk of non-compliance, incorrect versions, broken links, or inconsistent content, which can severely impact reviewer satisfaction and approval chances. * **Operational Challenges:** Beyond document processing, significant challenges include managing large file transfers (often 10-20+ GB per submission, requiring multiple transfers) and ensuring seamless integration of new submissions with previous ones. * **Strategic Compliance:** Proper eCTD planning and execution are not just technical tasks but strategic imperatives that directly influence regulatory approval timelines and outcomes by ensuring a clear, consistent, and easily reviewable application for regulatory bodies.

IDMP in a capsule Tutorial
UNICOM
/@UNICOM-IDMP
Mar 24, 2022
This. The emphasis on patient safety and pharmacovigilance further underscores its relevance to medical affairs and regulatory compliance departments within their target market. This tutorial provides a comprehensive overview of the Identification of Medicinal Products (IDMP) standards, highlighting their crucial role in ensuring global medication safety. It explains how the ISO IDMP standards, though inconsistently implemented, are designed to work throughout a medicinal product's life cycle, from development and production to utilization and outcome assessment. Through illustrative stories, the video demonstrates how IDMP helps prevent adverse drug events, facilitates safe substitutions across different countries, curbs falsified medicines, and enhances global pharmacovigilance. A central theme is the concept of Medicinal Product Dictionaries (MPDs) as national repositories for comprehensive product information, linked globally by the Pharmaceutical Product Identifier (PHP ID), enabling seamless data navigation and personalized patient care through integration with personal health data. Key Takeaways: * **Standardized Global Identification is Critical:** Inconsistent identification of medicinal products across countries poses significant patient safety risks. IDMP provides a standardized framework (substance, dose form, strength, medicinal product, package) to ensure globally unique identification, crucial for safe healthcare and preventing errors. * **Medicinal Product Dictionaries (MPDs) as Central Data Hubs:** MPDs are vital national repositories that consolidate comprehensive product information (regulatory, scientific, pricing, dosage guidance). These dictionaries are the foundation for all information needed by hospitals, doctors, and pharmacies for safe prescribing and dispensing, and are accessed through various healthcare IT systems. * **The PHP ID Enables Cross-Border Data Linkage:** The Pharmaceutical Product Identifier (PHP ID) is a critical global identifier that allows for the seamless navigation and comparison of equivalent medicinal products across different national MPDs. This capability is essential for facilitating safe substitutions for patients traveling internationally and for aggregating global pharmacovigilance data. * **IDMP Supports Personalized Patient Safety:** By integrating IDMP-compliant product data from MPDs with individual patient health data (e.g., International Patient Summary - IPS), intelligent applications can provide real-time, personalized alerts for allergies, intolerances, and potential drug-drug interactions, significantly enhancing medication safety at the point of care. * **Enhanced Global Pharmacovigilance:** IDMP-compliant reporting, which includes the PHP ID and other identifiers, streamlines the aggregation and analysis of adverse drug event data globally. This enables faster identification of safety patterns, quicker responses (e.g., product recalls, updated warnings), and proactive risk mitigation across populations. * **Call for Industry-Wide Implementation:** The full benefits of IDMP, including improved medication safety and public health outcomes, can only be realized through widespread and consistent implementation by pharmaceutical companies, regulators, and IT solution providers across the entire medicinal product life cycle.

An Industry Ontology for the Identification of Medicinal Products (IDMP)
Biomedical Ontology World
/@biomedicalontologyworld
Jul 18, 2022
This video introduces the Pistoia Alliance's IDMP Ontology project, which addresses the critical challenge of inconsistent interpretations of the ISO Identification of Medicinal Products (IDMP) standards within the pharmaceutical industry. Currently, IDMP standards are published as PDFs, leading to varied implementations across companies and regulatory agencies, hindering automated data processing, quality, and semantic interoperability. The project aims to augment these standards with a formal ontology, creating a semantically aligned and governed data model that acts as a "connecting bridge" for data interpretation across internal departments, suppliers, and regulators. The speaker emphasizes the importance of contextualizing complex concepts like "active moiety" to accommodate diverse scientific and regulatory perspectives, ensuring accurate representation while allowing for different expert views. The ontology is being developed collaboratively with major pharma companies and validated against real-world data from sources like FDA's GSRS, demonstrating its ability to answer specific competency questions and facilitate cross-organizational data integration for improved drug safety, innovation, and operational efficiency. Key Takeaways: * **Semantic Interoperability for Regulatory Compliance:** The IDMP Ontology directly tackles the lack of semantic alignment in pharmaceutical product data, a crucial step for achieving consistent regulatory compliance (FDA, EMA) and enabling automated data processing across the life sciences ecosystem. * **Ontologies as Foundational Data Bridges:** The project highlights the strategic value of ontologies in creating a unified "connecting bridge" for data interpretation, essential for harmonizing information flows within and between pharma companies, suppliers, and regulatory bodies. * **Contextualized Data Modeling for Complex Concepts:** The approach to modeling concepts like "active moiety" with contextual roles (e.g., regulatory, scientific, patent exclusivity) demonstrates a sophisticated method for managing inherent ambiguities and diverse expert perspectives in pharmaceutical data. This advanced data modeling is critical for developing robust custom software and AI solutions. * **Industry-Wide Collaborative Standard Setting:** The Pistoia Alliance's collaborative model, involving leading pharma companies (Bayer, Merck, GSK, Novartis, J&J) and regulatory agencies (FDA, EMA), underscores the industry's commitment to developing shared data standards that drive collective innovation, efficiency, and patient safety. * **Real-World Validation for Practical Application:** The project's emphasis on testing the ontology with actual data (e.g., FDA GSRS, pharma company data) and specific use cases ensures its practical applicability and demonstrates tangible value for solving real-world data integration and interpretation challenges, a key aspect of delivering effective consulting and software development.

🎓 Veeva Vault RIM Training | Regulatory Information Management Made Easy | Vistasparks Solutions
Vistasparks Solutions
/@Vistasparks-Solutions
Jul 6, 2025
This video from Vistasparks Solutions provides an in-depth overview of their training program for Veeva Vault RIM (Regulatory Information Management), a critical cloud-based platform for the life sciences industry. The training aims to equip professionals with the knowledge and tools to optimize regulatory operations by managing global submissions, registrations, correspondence, and commitments within a unified system. It covers both functional and technical aspects of Veeva Vault RIM, from foundational concepts like document management and interface navigation to advanced topics such as system administration, lifecycle configuration, dynamic access control, and system integration. The program emphasizes real-world application through case studies, hands-on practice in sandbox environments, and preparation for industry-recognized Veeva Vault RIM certification, ultimately preparing individuals for roles in regulatory affairs, IT consulting, and compliance within the pharmaceutical and biotech sectors. Key Takeaways: * **Unified Regulatory Information Management:** Veeva Vault RIM centralizes and streamlines the management of all regulatory assets, including global submissions, registrations, health authority interactions, and commitments, providing a single source of truth throughout the entire product lifecycle. * **Enhanced Compliance and Efficiency:** The platform significantly reduces manual effort (up to 30%) by automating key processes like commitment and submission management, ensuring adherence to regional regulatory standards, and providing robust compliance reporting and audit trails. * **Comprehensive Skill Development:** The training caters to a wide audience, from regulatory affairs professionals to IT consultants and compliance teams, offering a structured learning path that covers Veeva Vault fundamentals, advanced system administration, configuration, and integration skills. * **Practical Application and Certification:** A strong emphasis is placed on practical application through real-world use cases, hands-on exercises in simulated environments, and dedicated preparation for the Veeva Vault RIM certification exam, which is highly valued in the life sciences industry. * **Global Regulatory Focus:** The curriculum directly addresses the complexities of global regulatory operations, including health authority response management, planning and executing submissions across US, European, and Asia-Pacific markets, and managing post-approval commitments. * **Integration with Enterprise Ecosystems:** The training highlights Veeva Vault RIM's integration capabilities with other Veeva Vault modules (e.g., Quality and Clinical) and other systems, underscoring its role within a broader regulated enterprise software landscape.

Blockchain in Action: How IQVIA Built an App Streamlining Drug Labels
Salesforce Developers
/@SalesforceDevs
Jun 26, 2019
This video explores a real-world application of blockchain technology by IQVIA, a leading life sciences company, to streamline the complex and highly regulated process of managing prescription drug labels using Salesforce Blockchain. The speakers highlight how traditional methods for label changes are inefficient, involve numerous parties, and lack consistent version control, posing significant challenges for regulatory compliance and patient safety. Blockchain is presented as a transformative solution to these issues, offering an immutable ledger, distributed network, and consensus workflows to ensure the permanence, provenance, and real-time replication of critical label information across stakeholders, including pharmaceutical companies, regulatory bodies, and design teams. Key Takeaways: * **Blockchain for Regulated Content Management:** The video demonstrates a powerful application of blockchain in the pharmaceutical industry for managing highly sensitive and regulated content like drug labels, ensuring immutable evidence, auditability, and real-time consistency across a multi-party ecosystem. * **Addressing Operational Inefficiencies in Life Sciences:** Blockchain is positioned as a solution to overcome the inherent complexities and inefficiencies of traditional drug label management, which is characterized by disparate data sources, manual processes, and challenges in maintaining version control and audit trails. * **Architectural Paradigm Shift:** The speakers advocate for viewing blockchain as a new architectural layer for enterprise systems, specifically for recording immutable events and facilitating "collaborative coding" of smart contracts among different organizations, moving beyond its common association with supply chain or cryptocurrency. * **Enhanced Regulatory Compliance and Patient Safety:** By providing a trusted, shared record of all label changes and their history, the solution significantly enhances regulatory compliance (e.g., FDA, EMA, GxP) and enables rapid, secure updates to critical drug information, directly impacting patient safety. * **Salesforce's Role in Enterprise Blockchain:** The successful prototyping of this solution on Salesforce Blockchain underscores Salesforce's capability to provide a declarative, integrated platform for enterprise-grade blockchain applications, particularly within regulated industries. * **Data Provenance and Auditability:** The "blockchain Timeline view" feature, which tracks every change and its history, offers robust data provenance and audit trail capabilities, crucial for meeting stringent regulatory requirements.
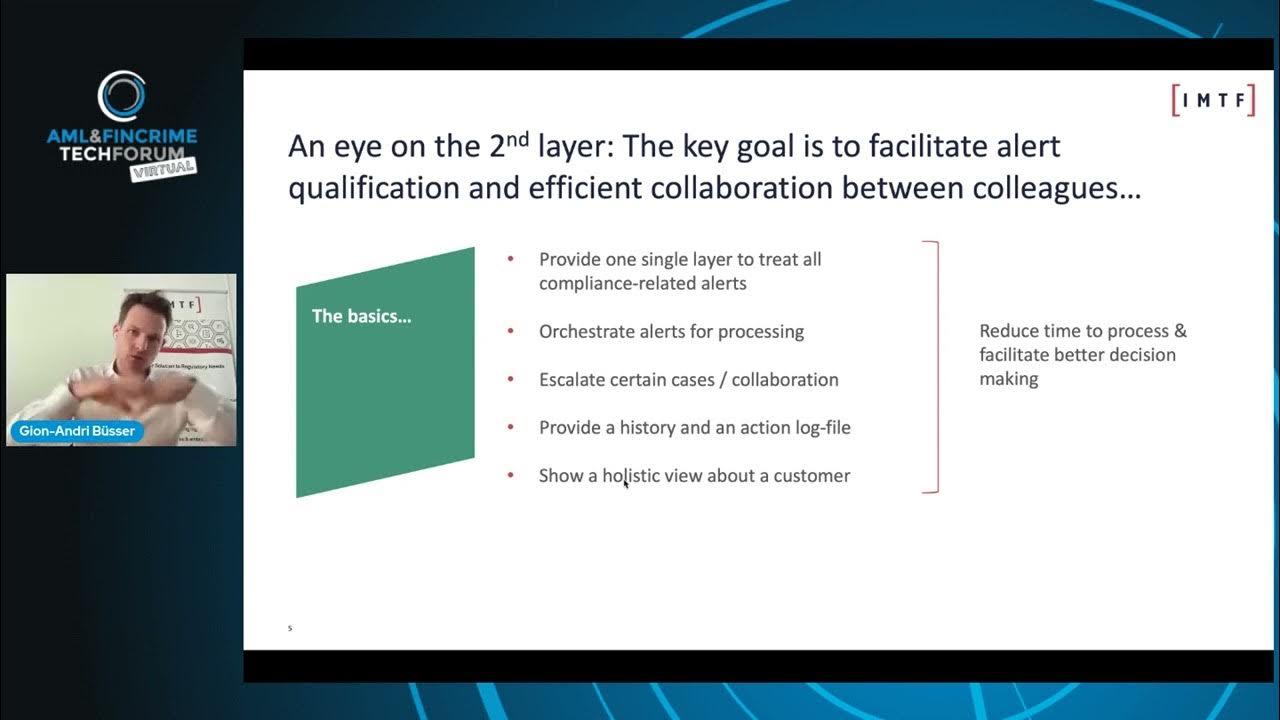
Benefits of a Two Layer Regulatory Intelligence Platform
IMTF - Excellence in RegTech Solutions
/@imtf-excellenceinregtech
Mar 11, 2022
The speaker, Gion-Andri Büsser of IMTF, discusses the critical need for modern technology in compliance, driven by exploding costs of compliance and even higher costs of non-compliance. He introduces a "two-layer regulatory intelligence platform" concept, emphasizing an orchestration layer that centralizes the treatment of compliance alerts, automates processes, fosters collaboration, and provides a holistic view of data. The video highlights how advanced technologies, including machine learning and data science, can be leveraged within this architecture to significantly reduce manual work, improve efficiency, and enhance risk management. Key Takeaways: * **Ecosystem Approach to Compliance Technology:** The video advocates for viewing compliance technology as an integrated ecosystem rather than disparate, siloed software packages. This platform approach is crucial for comprehensive data utilization and decision-making. * **The Power of the Orchestration Layer (Layer 2):** A dedicated orchestration layer is presented as essential for consolidating and processing all compliance alerts (e.g., AML, fraud, sanctions), enabling flexible process automation, fostering collaboration across teams, and maintaining accurate, regulator-ready audit trails. * **AI/ML for Enhanced Efficiency and Risk Reduction:** The "magic" of the orchestration layer lies in its ability to leverage AI and machine learning for advanced functions such as intelligent auto-closure of routine alerts, pre-processing to highlight historical patterns, and more granular, accurate risk rating based on comprehensive customer history. * **Strategic IT and Change Management Benefits:** Implementing an orchestration layer provides significant IT flexibility, allowing organizations to introduce modern tools and interfaces without immediately replacing existing, potentially valuable, legacy detection systems (Layer 1). This enables a phased modernization approach, reducing disruption and risk. * **Continuous Adaptation to Evolving Threats:** The speaker underscores that financial crime and compliance challenges are an "ever-ending battle," with criminal behaviors constantly evolving (e.g., due to pandemics, crypto). This necessitates adaptive, forward-looking technology to anticipate and combat new threats.g., GxP, 21 CFR Part 11, FDA/EMA regulations).

Veeva Vault RIM: Comprehensive Online Training learn from experts with proexcellency
Proexcellency Training
/@proexcellency_training
Aug 9, 2023
This video provides a comprehensive overview of a training program for Veeva Vault RIM, detailing its extensive functionalities for Regulatory Information Management within the pharmaceutical and life sciences industries. It covers the platform's role in streamlining compliance processes, managing regulatory documents and submissions (including IND, NDA, MAA), and ensuring regulatory success. The training curriculum explores key areas such as system configuration, user access control, document versioning, workflow automation, health authority communication, regulatory reporting, global alignment, and robust data integrity and security measures essential for compliance with standards like GxP and 21 CFR Part 11. The content underscores the platform's ability to enhance efficiency, collaboration, and reduce compliance risks across various regulatory activities Key Takeaways: * **End-to-End Regulatory Lifecycle Management:** Veeva Vault RIM offers comprehensive capabilities for managing the entire regulatory information lifecycle, from initial document authoring and submission planning to dossier management, publishing, and long-term archiving, which is crucial for pharmaceutical and life sciences companies. * **Enhanced Collaboration and Data Integration:** Veeva Vault RIM facilitates cross-functional collaboration, enabling efficient information sharing and integration of regulatory data with other critical departments such as Quality Assurance and Clinical Operations, streamlining overall operational workflows. * **Strategic Reporting and Global Alignment:** The system provides powerful reporting and analytics tools for tracking submission status, compliance trends, and key performance indicators. It also supports global regulatory alignment, helping organizations manage submissions and adhere to diverse international regulatory standards. * **Risk Management and Continuous Improvement:** Veeva Vault RIM supports proactive regulatory risk management through impact analysis for changes and fosters continuous improvement by identifying and addressing process inefficiencies, contributing to sustained regulatory excellence.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Nov 29, 2021
This RegTalks episode, featuring Lidia Canovas of Asphalion and Udo Griem of Körber Pharma Software, provides an in-depth discussion on Regulatory Information Management Systems (RIMS) within the pharmaceutical and biotech industries. The speakers emphasize the critical role of RIMS in managing medicinal product data, particularly in anticipation of the complex transition to ISO IDMP standards. Griem defines RIMS through three core pillars: a solid, data-oriented database serving as a single source of truth for product master data, a robust workflow engine to manage submissions and approval processes, and capabilities for structured data submissions to authorities. He advocates for a data-oriented approach over traditional document-driven methods to ensure data quality and consistency. Key drivers for RIMS implementation include the impending IDMP mandate, the industry's shift towards structured data, competitive pressures for faster time-to-market, and the need for organizational process optimization. RIMS are shown to be beneficial for companies of all sizes and product portfolios, fostering a culture of quality and improving cross-departmental collaboration. While regulatory affairs is the primary user, RIMS serve as a central hub, supporting cross-functional processes across clinical, ERP, safety/pharmacovigilance, MES, and label artwork management. A significant emerging trend highlighted is the connection of RIMS data to data lakes for future evaluation with artificial intelligence. The discussion also underscores the importance of experienced implementation partners who can bridge the gap between software solutions and organizational needs, ensuring successful deployment and continuous improvement. Körber's AI Manager RIMS aims to minimize compliance risks, enhance efficiency, enable teamwork, and reduce costs, with IDMP compliance being a high priority in its development roadmap. **Key Takeaways:** * **RIMS are Foundational for Regulatory Compliance and Efficiency:** RIMS are critical for managing complex regulatory data, streamlining submissions, and ensuring compliance with evolving standards like IDMP, which is rapidly approaching and will significantly increase data complexity. * **Data-Oriented Approach is Paramount for RIMS:** A consistent, data-oriented design with a well-organized database is considered superior to document-driven approaches, ensuring high data quality, consistency, and adaptability for future regulatory requirements. * **RIMS Drive Cross-Functional Integration and Quality Culture:** Beyond regulatory affairs, RIMS act as a central hub, connecting and supporting various departments (e.g., clinical, ERP, safety, MES, QA) to improve collaboration, reduce error rates, and shorten response times across the pharmaceutical value chain. * **AI Integration is an Emerging Trend for RIMS Data:** There is a growing focus on connecting RIMS data to data lakes, specifically for evaluation with artificial intelligence, indicating a future direction for leveraging regulatory information for advanced insights and automation. * **Implementation Partners are Crucial for Successful RIMS Adoption:** Experienced partners with deep regulatory and product knowledge are vital for guiding companies through organizational and process changes, customizing solutions, and ensuring successful integration and continuous improvement. * **RIMS Benefits Extend to All Company Sizes and Diverse Portfolios:** While historically adopted by large multinationals, RIMS are now essential for small and mid-tier firms, including startups, to effectively build and scale regulatory organizations and manage diverse product portfolios beyond human medicine.

Mastering Change Management Veeva RIM Implementation Case Study
Astrix On Demand Webinars for Life Sciences
/@astrixlifescience
Nov 26, 2024
This video explores the critical role of Organizational Change Management (OCM) in ensuring successful technology implementations, particularly within the life sciences industry. The speaker details OCM's core principles—communication, training, stakeholder engagement, and continuous improvement—and explains why it is essential for enhancing adaptability, reducing resistance, boosting productivity, mitigating risks, and aligning with strategic goals. The presentation culminates in a case study of a global Veeva Regulatory Information Management (RIM) module implementation, demonstrating how a structured OCM framework can lead to successful adoption, improved regulatory processes, enhanced compliance, and streamlined documentation across diverse stakeholder groups. Key Takeaways: * The video provides a detailed overview of Organizational Change Management (OCM) principles, emphasizing its critical role in successful technology adoption within the life sciences sector. * It highlights OCM's importance in enhancing adaptability, reducing resistance, boosting productivity, mitigating risks, and ensuring strategic alignment for complex implementations. * A key focus is on a real-world case study involving the global implementation of Veeva Regulatory Information Management (RIM) modules * The case study demonstrates how tailored strategies, comprehensive communication plans, stakeholder engagement, and continuous improvement are essential for achieving regulatory process enhancement, improved compliance, and streamlined documentation. * Common resistance points (e.g., fear of the unknown, loss of control, increased workload) are addressed with practical strategies, offering valuable insights for managing the "people side of change" during software rollouts. * Success measurement in OCM is detailed through metrics like team engagement, communication effectiveness, training completion, adoption rates, and performance outcomes, providing a framework for evaluating implementation success.

2022 RDCA-DAP Workshop: Case Study 3, Duchenne Regulatory Science Consortia (D-RSC)
Critical Path Institute
/@CriticalPathInstitute
Oct 5, 2022
This video presents a case study from the Duchenne Regulatory Science Consortia (D-RSC) by the Critical Path Institute, focusing on efforts to accelerate therapeutic development for Duchenne Muscular Dystrophy (DMD). Speaker Terina Martinez highlights the significant challenges in DMD drug development, including high clinical trial failure rates, disease complexity, and difficulties in patient selection and endpoint measurement. To address these issues, D-RSC has developed a Clinical Trial Simulation (CTS) tool. This tool leverages a rich, integrated database of patient-level data from numerous clinical trials and natural history studies to train and validate quantitative disease progression models. The goal is to enable in silico evaluation and refinement of clinical trial designs, optimizing parameters like sample size and duration, and simulating drug effects. The tool is undergoing regulatory qualification with both the EMA and FDA, emphasizing its rigor and potential to improve trial efficiency, reduce participant burden, and stimulate further data sharing within the rare disease community. Key Takeaways: * **Data-Driven Clinical Trial Optimization:** The D-RSC's Clinical Trial Simulation (CTS) tool exemplifies how aggregating and analyzing extensive patient-level data from clinical trials and natural history studies can be leveraged to create quantitative disease progression models. This enables in silico evaluation and optimization of trial designs for rare diseases like DMDai. * **Regulatory Qualification of Novel Methodologies:** The active pursuit of regulatory qualification for the CTS tool through both EMA's Qualification of Novel Methodologies and FDA's Fit for Purpose Initiative highlights the growing importance of formally validating advanced computational tools for use in drug development and decision-making. This directly * **Impact on Trial Efficiency and Data Sharing:** Such simulation tools are crucial for improving clinical trial efficiency by optimizing sample size, trial duration, and patient selection, thereby reducing the burden on rare disease patient populations. Regulatory approval also acts as a strong catalyst for increased data sharing among stakeholders, underscoring the value of robust data integration and management. * **Addressing Rare Disease Challenges with Technology:** The initiative directly tackles the unique complexities of rare disease drug development, including high failure rates, heterogeneity in disease progression, and the need for better clinical endpoints, by providing a data-driven approach to de-risk and accelerate therapeutic advancement.

NNIT Webinar: IDMP enforcement in Europe in 2021
NNIT | We make a mark
/@NNITvideo
Jan 25, 2021
This video provides a critical update on the European Medicines Agency's (EMA) Identification of Medicinal Products (IDMP) initiative, focusing on its enforcement timelines and the latest developments in the implementation guide. The speakers, regulatory affairs experts from NNIT, detail the expected publication of IDMP Implementation Guide Version 2 on February 22, 2021, which sets the mandatory go-live date for PMS (Product Management System) Iteration 1 fields for the industry by February 22, 2023. The discussion highlights the significant impact on regulatory affairs, the need for comprehensive data management and quality, and the ongoing evolution of EMA's approach to IDMP, including an agile documentation strategy and updates on substance data management. Key Takeaways: * **Imminent IDMP Enforcement:** The EMA has set a concrete timeline for IDMP enforcement, with PMS Iteration 1 becoming mandatory for industry by February 22, 2023, following the publication of Implementation Guide Version 2. This signals a definitive move towards compliance after years of delays. * **"One Side IDMP, Always IDMP" Mandate:** An unofficially confirmed but critical rule states that once a company submits IDMP information for one product, all other products in its portfolio must subsequently follow suit. This eliminates the possibility of small-scale pilots and necessitates a comprehensive, portfolio-wide IDMP strategy from the outset. * **Holistic Data & Process Transformation:** IDMP compliance extends beyond IT systems, requiring significant organizational effort in locating and ensuring the quality of required data, as well as fundamental changes to business processes across regulatory, commercial, and potentially clinical operations. * **Evolving Substance Data Management:** The EMA is actively cleansing and synchronizing substance data through its EU SRS and SMS projects, with a focus on aligning with the FDA's GSRS. This indicates a push towards greater international harmonization in substance identification and management. * **Limitations of Standard RIM Systems:** While many Regulatory Information Management (RIM) system providers are incorporating IDMP roadmaps, their solutions primarily address data model expansion and basic functionality. They often do not cover critical aspects like comprehensive data quality monitoring, integration with other enterprise systems, or the necessary adjustments to overall submission processes, highlighting the need for broader data engineering and process re-design efforts. * **Strategic Industry Preparation is Crucial:** Companies must proactively prepare by staying updated on regulatory changes, mapping substances and referentials, and raising internal awareness. It's essential to plan for all product types (centralized, mutual recognition, decentralized, and national procedures) from the beginning, rather than focusing solely on centralized products.

Is this Stock Better Than Salesforces Inc? | Veeva Systems Inc (NYSE:VEEV)
Learn With Stanley
/@learnwithstanley
Aug 11, 2022
This video provides an investment analysis of Veeva Systems Inc., detailing its origins, core offerings, market dominance, and growth trajectory within the life sciences industry. The speaker explains how Veeva was founded to address the specific cloud-based CRM needs of life sciences companies, which were not adequately met by general platforms like Salesforce. The discussion highlights Veeva's two main ecosystems: the Commercial Cloud, providing CRM and analytical services, and Veeva Vault, a suite of applications for managing clinical trials, industrial regulations, and other critical data points. The video underscores Veeva's position as the de facto cloud service provider for the global life sciences sector, enabling drug companies to develop products, enhance sales and marketing, and ensure regulatory compliance. It also covers Veeva's impressive customer growth, high revenue retention rates, and future revenue projections, attributing its success to a first-mover advantage and the continuous demand for specialized solutions in a highly competitive market. Key Takeaways: * **Specialized Industry Focus:** Veeva Systems' success stems from its targeted approach to the life sciences industry, demonstrating the critical need for specialized cloud-based CRM and data management solutions beyond generic enterprise platforms. * **Comprehensive Platform for Life Sciences:** Veeva offers two key ecosystems: the Commercial Cloud for CRM and analytics, and Veeva Vault for managing clinical trials, regulatory compliance, and other crucial data, covering a broad spectrum of pharmaceutical operations. * **Market Dominance and Entrenchment:** Veeva is identified as the "de facto cloud service provider" for the global life sciences industry, indicating a strong first-mover advantage and significant market share in a highly regulated and specialized sector. * **Addressing Core Industry Needs:** Veeva's solutions directly support drug companies in three vital areas: effective product development and market entry, efficient marketing and sales to healthcare professionals, and maintaining strict compliance with industry and government regulations. * **High Customer Retention and Growth Drivers:** The company's impressive customer growth (over 1,000 customers including major pharma giants) and high revenue retention rates (around 120%) reflect the ongoing demand for its services, driven by intense competition and regulatory requirements within life sciences.
![The Good, the Bad, and the Ugliness of Regulation [No. 86 LECTURE]](https://i.ytimg.com/vi/FgTRVi23LGo/maxresdefault.jpg)
The Good, the Bad, and the Ugliness of Regulation [No. 86 LECTURE]
The Federalist Society
/@TheFederalistSociety
Feb 23, 2023
This video, "The Good, the Bad, and the Ugliness of Regulation," features Professor Susan Dudley discussing the origins, functions, and impact of administrative agencies and regulations in the U.S. It delves into the historical context of administrative law, tracing the evolution from early economic regulations to the modern prevalence of social regulations concerning environment, health, and safety. Professor Dudley explains the constitutional debates surrounding agency powers, the significance of the Administrative Procedure Act, and various methods for measuring the vast scope and economic impact of regulations. She highlights that while regulations are often perceived as burdensome, they are fundamentally justified by market failures such as externalities and asymmetric information, which are particularly relevant in complex industries like pharmaceuticals and life sciences. Key Takeaways: * **Foundational Role of Agencies:** Administrative agencies emerged to address the increasing complexity of society, operating under legislative delegation (e.g., Administrative Procedure Act of 1946) to create detailed regulations that Congress lacks the expertise or capacity to write. * **Shift to Social Regulation:** While early regulation focused on economic aspects, there has been a dramatic and continuing increase in "social regulation" since the 1970s, covering areas like environment, health, safety, and workplace conditions. This category directly encompasses the regulations critical to the pharmaceutical and life sciences industries. * **Market Failures as Justification:** The primary rationale for regulation stems from market failures, including externalities (costs borne by third parties, like pollution) and asymmetric information (where one party lacks crucial information, such as product safety or efficacy). * **Vast and Growing Regulatory Landscape:** There are at least 100 federal agencies issuing thousands of regulations annually, with 50-100 "economically significant" regulations (>$100 million impact) each year.ai helps its clients manage through AI and consulting services. * **Challenges in Measuring Regulatory Impact:** Unlike fiscal budgets, there's no single, comprehensive measure for the total impact of regulations, making it difficult for businesses to fully grasp and comply with the "stock" of existing rules (Code of Federal Regulations) and the "flow" of new ones (Federal Register). This complexity highlights the value of specialized consulting and AI solutions for compliance tracking.

RIM Implementation in times of IDMP - Asphalion Webinar
Asphalion
/@Asphalion.
Jul 26, 2022
This video explores the critical intersection of Regulatory Information Management (RIM) system implementation and the ongoing transition to ISO Identification of Medicinal Products (IDMP) standards. The speaker details the current uncertainties surrounding IDMP, the phased rollout of EMA's SPOR project (including PMS and the DADI web form), and the profound impact these changes have on the pharmaceutical industry. A central theme is how RIM systems serve as essential tools to navigate IDMP compliance, emphasizing the need for digitalization, robust data governance, and strategic data migration during implementation. Key Takeaways: * **IDMP as a Digitalization Catalyst:** ISO IDMP is presented not merely as a regulatory requirement but as a significant driver for comprehensive digitalization and the establishment of strong data governance frameworks within pharmaceutical companies, necessitating standardized and interoperable data. * **RIM Systems are Core for IDMP Compliance:** Regulatory Information Management (RIM) systems are highlighted as indispensable for IDMP compliance, facilitating efficient data collection, organization, and communication. Future RIM systems are expected to directly support data submission to EMA's PMS via fire messages, evolving beyond current xEVMPD processes. * **Data Quality and Governance are Foundational:** Successful IDMP compliance and RIM implementation are contingent upon high-quality, harmonized data. Companies must prioritize data cleaning, enrichment, and mapping, integrating IDMP requirements from the initial planning stages of any RIM project. * **Navigating Phased IDMP Rollout Complexity:** The EMA's phased implementation of IDMP through the DADI web form (starting with Centrally Authorized Products (CAPs), then Nationally Authorized Products (NAPs)) introduces a complex transitional period. Organizations must plan to manage the coexistence of legacy and new systems and processes effectively. * **Strategic Implementation is Crucial:** Regardless of company size or portfolio, a well-defined strategy for IDMP compliance and RIM implementation is vital. This involves a thorough assessment of existing data structures, internal processes, technological capabilities, and future expectations to select the most compatible RIM system and implementation approach.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Nov 5, 2021
This video explores the critical role of Regulatory Information Management Systems (RIMS) within the pharmaceutical industry, particularly in light of the upcoming transition from XEVMPD to the more complex ISO IDMP standard. Featuring insights from Veeva's Director of Strategy for Vault RIM, Katrin Spaepen, the discussion highlights the drivers for RIMS adoption, essential capabilities of future systems, and the multi-departmental nature of their use. The speakers emphasize that RIMS are key to managing vast amounts of regulatory data, ensuring compliance, and optimizing operations for medicinal products. Key Takeaways: * **Strategic Importance of RIMS:** RIMS are fundamental for data management, operational efficiency, and maintaining regulatory compliance in the pharmaceutical industry, especially with the impending, more complex ISO IDMP standard. * **Drivers for RIMS Adoption:** Companies adopt RIMS for three primary reasons: driving enterprise-wide digital transformation, achieving process efficiencies and cost optimizations in regulatory domains, or ensuring compliance (often driven by mandates like IDMP). These drivers influence the scope and approach to implementation. * **Core Capabilities of Future RIMS:** Effective RIMS must offer native support for both data and documents, ensure synchronization between them, feature an open architecture for data exchange with non-RIM systems, provide a complete view of license data across the enterprise, and embed real-time reporting and business intelligence dashboards. * **Multi-Departmental Utility:** RIMS are not solely for regulatory affairs; they serve as an enterprise-wide solution involving various departments such as regulatory operations, CMC writers, medical writers, manufacturing, quality control, and safety, particularly in processes like post-approval variation management. * **Crucial Role of Implementation Partners:** Successful RIMS implementation necessitates strong partnerships for process re-engineering, organizational impact assessment, change management, gap analysis, and defining key performance indicators (KPIs) and user requirements (URS). * **Veeva's Market Presence:** Veeva's Vault RIM suite (encompassing submissions, archive, publishing, and registration) is positioned as a leading end-to-end solution supporting the full regulatory process, indicating its significance in the industry.

Automating IDMP preparation with PhlexGlobal
Informa Connect Life Sciences
/@Ibclifesciences
Mar 11, 2021
This video provides an in-depth exploration of how artificial intelligence and machine learning are being applied to automate regulatory processes within the pharmaceutical industry, with a particular focus on preparing for the Identification of Medicinal Products (IDMP) mandate. Jim Nichols, Chief Product Officer at Phlexglobal, discusses the company's "Flex Neuron" AI technology and its role in transforming document-driven regulatory operations into more efficient, data-driven processes. The discussion highlights the critical shift in regulatory compliance from unstructured content to structured data, emphasizing the strategic value this transformation brings to pharmaceutical companies. The core of the conversation revolves around the "datification of regulatory," a trend driven by global health authorities like the EMA and FDA. Phlexglobal's Flex Neuron platform leverages AI and machine learning to extract, classify, and prepare data from various regulatory documents, such as SmPCs (Summary of Product Characteristics) and Module 3 documents. This automation is crucial for populating data management solutions like Flex IDMP, ensuring that pharmaceutical companies are ready for upcoming mandatory submissions. The video also details how AI assists in Trial Master File (TMF) management by automating document indexing and placement, significantly reducing manual effort and enhancing accuracy. Nichols provides concrete examples, including Phlexglobal's work with AstraZeneca, where AI has been used to recursively extract IDMP data from regulatory documents and to automate the cataloging and metadata population of health authority communications into systems like Veeva Vault. He underscores that IDMP is currently the most significant regulatory update, with the recent release of Implementation Guide v2 initiating a two-year countdown to mandatory submissions. The ultimate goal of IDMP, as part of EMA's Article 57, is to enhance patient safety through a comprehensive, hierarchical collection of data that enables better impact analysis and signal detection for adverse events. For pharmaceutical companies, this transition means regulatory information evolves from a compliance burden into a valuable strategic asset, offering greater visibility into their product portfolios and facilitating proactive decision-making. Key Takeaways: • The "datification of regulatory" is a transformative trend in the pharmaceutical industry, shifting focus from unstructured documents to structured data, driven by global health authorities like the EMA and FDA. This fundamental change is vital for modernizing compliance and leveraging regulatory information strategically. • IDMP (Identification of Medicinal Products) is identified as the most critical new regulation, with the recent release of Implementation Guide v2 starting a two-year countdown to mandatory data submissions, requiring urgent preparation from pharmaceutical companies. • Artificial intelligence and machine learning, exemplified by Phlexglobal's Flex Neuron, are essential for automating the extraction and encoding of structured data from vast volumes of unstructured regulatory documents (e.g., SmPCs, Module 3 documents) to meet IDMP requirements. • Automation significantly improves Trial Master File (TMF) management through AI-assisted document indexing and classification, which reduces labor-intensive manual processes, ensures proper attribution, and accurately places documents within the TMF reference model structure. • Transitioning from document-driven to data-driven processes enables pharmaceutical companies to perform more effective analytics, conduct impact analyses, and enhance signal detection for patient safety, ultimately leading to better product lifecycle and regulatory management. • Structured regulatory information transforms from a mere compliance overhead into a strategic asset, providing pharmaceutical companies with valuable internal visibility into their products and enabling proactive decision-making regarding product changes and regulatory impacts. • Companies should identify high-volume document areas where critical data is currently "trapped" to prioritize and target automation efforts effectively, focusing on problems that offer the biggest value return for AI implementation. • AI solutions can be integrated with existing content management systems, such as Veeva Vault, to automate the cataloging, classification, and metadata population of incoming health authority communications, streamlining regulatory operations. • Companies currently compliant with xEVMPD (Extended EudraVigilance Medicinal Product Dictionary) must understand the migration path to IDMP and be prepared to operate in both worlds simultaneously, requiring robust bridging mechanisms to maintain compliance during the transition. • The EMA's ultimate goal for IDMP, under Article 57, is to enhance patient safety by centralizing detailed, hierarchical product data, enabling more sophisticated impact analysis and signal detection for adverse events at a granular level. • Pharmaceutical companies benefit from IDMP by gaining enhanced internal visibility into their own products, allowing them to conduct more precise impact analyses on product changes and better understand how regulatory and product lifecycles affect their operations. • Regulatory Information Management (RIM) encompasses IDMP, emphasizing the need for robust connectivity across all regulatory activities, ensuring that actions like filing a Type 2 variation are seamlessly tied to corresponding IDMP submissions. • Phlexglobal offers comprehensive support for IDMP readiness, including data extraction services using Flex Neuron, intuitive data management applications, and analytical services to help companies map and aggregate data from various internal sources. Tools/Resources Mentioned: * **Flex Neuron:** Phlexglobal's AI/ML technology for content extraction, document classification, and data preparation. * **Flex TMF:** Phlexglobal's Trial Master File software, which embeds Flex Neuron for AI-assisted document indexing. * **Flex IDMP:** Phlexglobal's data management solution for staging and managing IDMP data. * **Flex xEVMPD:** Phlexglobal's solution for xEVMPD submissions, serving as a precursor to IDMP. * **Veeva Vault:** A content management system mentioned as being integrated with AI for categorizing health authority communications. Key Concepts: * **IDMP (Identification of Medicinal Products):** International standards for the unique identification and exchange of medicinal product information, mandated by the EMA. * **xEVMPD (Extended EudraVigilance Medicinal Product Dictionary):** A precursor to IDMP, used for submitting medicinal product data to the EMA. * **TMF (Trial Master File):** A collection of essential documents that individually and collectively permit the evaluation of the conduct of a clinical trial and the quality of the data produced. * **RIM (Regulatory Information Management):** The systematic management of all regulatory data, documents, and processes throughout the product lifecycle. * **Datification of Regulatory:** The industry trend of transforming unstructured regulatory content into structured, actionable data for improved compliance, analytics, and strategic decision-making. * **AI-assisted Document Indexing:** Using artificial intelligence to automatically suggest or apply metadata and classification to documents, reducing manual effort and improving accuracy. * **Structured Product Labeling (SPL):** An FDA-regulated standard for exchanging product and establishment information. * **PQ CMC Initiative (Product Quality and Chemistry, Manufacturing, and Controls):** An FDA initiative aimed at modernizing CMC data submission and review. * **Structured Product Monograph:** A Health Canada initiative for structured product information. * **Electronic Prescribing Information (ePI):** A European initiative to transition from paper-based prescribing information to structured electronic formats. Examples/Case Studies: * **AstraZeneca:** Phlexglobal assisted AstraZeneca in preparing for IDMP by recursively extracting IDMP data from numerous regulatory documents, quality checking it, and providing an up-to-date data collection for their IDMP management system. * **Health Authority Communications:** Phlexglobal worked on a project where approximately 15,000 health authority communications per year needed to be cataloged into a content management system (Veeva Vault). Their AI system was trained to identify attributes like product, agency, and registration, pre-populating metadata for proper categorization and classification.

What is Veeva Vault RIM? | Implementation of Veeva Vault RIM into a Life Science Company
kyyah abdul
/@kyyahabdul
Jul 5, 2022
This video provides an in-depth exploration of Veeva Vault RIM, a critical cloud-based platform designed to streamline global regulatory processes within life science companies. The speaker, a regulatory professional, offers a user-centric perspective, demystifying the platform's functionalities and highlighting its transformative impact on document management and regulatory compliance. She emphasizes that while Veeva's official definition might seem convoluted, Vault RIM essentially functions as a comprehensive, centralized document management system for all regulatory-related documents within an organization. The presentation delves into the core components of the Veeva Vault RIM suite, which includes the Vault Platform, Vault Quality Docs, Vault Regulations, and Vault Submissions. The speaker contrasts this integrated solution with the fragmented and often inefficient systems previously used in the industry, such as Liquid Insight, Rosetta Phoenix, GDMRS, and even basic tools like Google Docs. She recounts personal experiences where smaller companies struggled with quality control and version management due to disparate applications and manual processes, particularly for large regulatory documents like protocols or briefing books. Veeva Vault RIM addresses these challenges by consolidating various tools—from housing documents and SOPs to building the eCTD backbone—into a single, unified application. A significant portion of the video is dedicated to explaining the practical application and workflow benefits of Veeva Vault RIM. The speaker details how the platform facilitates critical regulatory processes, such as authoring, pre-publishing, and final approval of documents. She illustrates an "authoring workflow" where medical writing initiates a document for review, allowing multiple stakeholders to collaborate simultaneously within the document while maintaining stringent version control. The system ensures that only changes made directly within the Vault are reflected, preventing issues arising from downloaded or externally modified files. Once a document is approved, it becomes locked, and any subsequent changes trigger an "up-versioning" process (e.g., from version 1.0 to 1.1 or 2.0), creating a clear audit trail. The platform also allows for flexible categorization of documents, typically by product, ensuring all related submissions and materials are easily accessible. The speaker also notes its capability to manage quality documents and SOPs for training, similar to specialized systems like MasterControl, but within a broader, integrated framework. Key Takeaways: * **Veeva Vault RIM's Core Purpose:** Veeva Vault RIM is a cloud-based platform that centralizes and streamlines global regulatory processes for pharmaceutical and life science companies, enhancing visibility, data quality, and agility in document management. * **Consolidated Document Management:** The platform serves as a comprehensive document management system, housing all regulatory documents, SOPs, and related materials in one unified application, eliminating the need for multiple disparate tools. * **Addressing Legacy System Challenges:** It solves common problems faced with older systems like Liquid Insight, Rosetta Phoenix, GDMRS, or even Google Docs, which often led to poor version control, quality inconsistencies, and workflow inefficiencies, especially for large regulatory documents. * **Integrated Suite Components:** The Veeva Vault RIM suite comprises several key tools, including the Vault Platform, Vault Quality Docs, Vault Regulations, and Vault Submissions, offering a holistic solution for various regulatory needs. * **Streamlined Regulatory Workflows:** The platform supports end-to-end regulatory workflows, from initial document authoring and collaborative review to pre-publishing formatting and final approval processes, ensuring a structured and controlled progression. * **Robust Version Control:** A standout feature is its advanced version control system, which allows multiple users to edit documents simultaneously while ensuring all changes are tracked and saved within the system, preventing external modifications from disrupting the official record. * **"Up-Versioning" for Audit Trails:** Once a document is approved, it becomes locked. Any subsequent changes automatically trigger an "up-versioning" (e.g., from 1.0 to 1.1 or 2.0), providing a clear, auditable history of all modifications and approvals. * **Broad User Adoption:** Veeva Vault RIM is widely used by regulatory professionals across all levels, from entry-level associates to senior managers, underscoring its essential role in daily operations within life science companies. * **Flexible Document Categorization:** The platform allows companies to categorize documents effectively, typically by product, making it easy to locate all submissions and materials related to a specific therapeutic area or product line. * **Quality Document Management:** Beyond regulatory submissions, Vault RIM also functions as a quality document management tool, capable of housing SOPs and supporting training processes, similar to specialized systems like MasterControl. * **Organizational Adoption Considerations:** While highly beneficial, smaller companies (5-15 people) might find the initial expense of a Veeva system prohibitive. Organizations are advised to inquire about informational sessions as they grow to determine if it's a suitable investment. * **Career Pathway for Individuals:** For individuals interested in careers in submissions management or regulatory operations, understanding and gaining proficiency in Veeva Vault RIM is invaluable, as it is a prevalent tool in the industry. **Tools/Resources Mentioned:** * Veeva Vault RIM * Veeva Vault Platform * Veeva Vault Quality Docs * Veeva Vault Regulations * Veeva Vault Submissions * Liquid Insight (legacy tool) * Rosetta Phoenix (legacy tool) * GDMRS (Global Document Regulatory System - legacy tool) * Google Docs (legacy tool for small companies) * MasterControl (quality document management system, compared to Vault Quality Docs) **Key Concepts:** * **Regulatory Information Management (RIM):** The systematic process of managing and tracking all information and documents related to regulatory submissions, approvals, and compliance throughout the product lifecycle in the life sciences. * **Cloud-based Platform:** A software system hosted on the internet, accessible from anywhere, offering scalability and centralized data management. * **Version Control:** A system that manages changes to documents over time, allowing users to track revisions, revert to previous versions, and ensure document integrity. * **eCTD Backbone:** The electronic Common Technical Document structure, a standardized format for submitting regulatory information to health authorities, which Veeva Vault RIM helps build and manage. * **Authoring Workflow:** A structured process for creating and reviewing documents, involving multiple contributors and reviewers, with defined roles and deadlines. * **Pre-publishing Workflow:** The stage where documents are formatted, checked for compliance with submission standards, and prepared for final submission. * **Approval Process:** The final stage in document management where designated authorities review and formally approve a document, often locking it from further changes without a new version. * **Quality Documents (QDocs):** Documents related to a company's quality management system, such as Standard Operating Procedures (SOPs), quality manuals, and training records.

Veeva Vault RIM Overview : Submission Key Features.
Anitech Talk
/@AnitechTalk
Aug 19, 2023
This video provides an in-depth exploration of Veeva Vault RIM (Regulatory Information Management), specifically focusing on its submission key features. The speaker, presenting on "Day 2" of a series following a discussion on the RIM data model, aims to deliver valuable insights to professionals working with Veeva applications. The core objective is to demystify the submission process within Veeva Vault RIM, highlighting how its functionalities streamline regulatory content management from authoring to approval and assembly. The presentation begins by outlining the fundamental structure of the Veeva Vault RIM application, which is characterized by two primary components: 'Submission' and 'Regulatory Objective.' The speaker clarifies that a regulatory objective represents a goal that must be achieved by submitting content to a health authority, with the submission itself being the mechanism to fulfill this objective. The discussion then transitions into the core features designed for efficient management of regulatory content, including content management, submission templates, a customizable data model, and robust review workflows. A significant portion of the explanation is dedicated to the eCTD (Electronic Common Technical Document) format, which is the standard followed by regulatory bodies like the FDA, detailing its five-module structure and the unique purpose of each module, such as Module 3 for quality documents and Module 5 for clinical study reports. Further into the video, the speaker elaborates on specific key features that enhance the submission process. These include predefined document types (e.g., clinical, non-clinical, quality, regulatory, pharmacovigilance) that align with submission requirements, and a comprehensive lifecycle and workflow system that guides submissions through various states like "planning," "send for health authority," and "authority received." A notable improvement highlighted is the content planning feature, which allows users to create detailed lists of documents for submission directly within Veeva Vault RIM, replacing traditional spreadsheet-based methods. The video also touches upon the "report level content plan" for publishing content outside of a main submission and the "publishing status indicator," a visual cue (green sign) that confirms a content plan is ready for publication after backend validation. The speaker emphasizes how these integrated features contribute to a seamless and compliant experience for regulatory professionals. Key Takeaways: * **Veeva Vault RIM Structure:** The application is fundamentally built around two interconnected components: 'Submission' and 'Regulatory Objective.' A submission is the means by which a regulatory objective (a goal to be achieved with a health authority) is fulfilled. * **End-to-End Submission Management:** Veeva Vault RIM is designed as an end-to-end application for the efficient management of regulatory content, encompassing authoring, review, approval, and assembly of various submission types. * **Core Submission Features:** Key functionalities include robust content management with pre-configured document types, submission templates adhering to industry standards, a customizable data model, and tailored review workflows to ensure compliance. * **eCTD Format Adherence:** The system supports the eCTD (Electronic Common Technical Document) format, which is a recommended structure for regulatory submissions, particularly for health authorities like the FDA. * **eCTD Module Breakdown:** The eCTD structure comprises five distinct modules, each serving a unique purpose; for example, Module 3 is dedicated to quality documents, Module 4 to non-clinical study reports, and Module 5 to clinical study reports. * **Pre-configured Document Types:** Veeva Vault RIM offers default document types, subtypes, and classifications (e.g., clinical, non-clinical, quality, regulatory, pharmacovigilance) that are pre-aligned with common regulatory submission requirements, simplifying content organization. * **Lifecycle and Workflow Management:** Submissions are guided through defined lifecycle states and workflows (e.g., "planning," "send for health authority," "authority received") to ensure proper progression, tracking, and adherence to regulatory processes. * **Streamlined Content Planning:** The platform includes a dedicated content planning feature that allows users to quickly create and manage lists of all documents expected for a submission, effectively replacing manual spreadsheet-based planning and ensuring alignment with eCTD format. * **Report Level Content Plans:** Beyond main submissions, Veeva Vault RIM supports "report level content plans," enabling the publishing of specific content or reports independently, catering to diverse regulatory needs. * **Publishing Status Indicator:** A crucial feature for publishers is the "publishing status indicator," which provides a clear visual confirmation (e.g., a green sign) that a content plan and its associated documents have successfully completed backend validation and are ready for publication or import. * **Tailored Processes for Compliance:** The customizable data model and review workflows allow organizations to tailor processes to their specific needs, ensuring compliance with regulatory standards and internal operational requirements. * **Seamless User Experience:** The integrated nature of these features within Veeva Vault RIM aims to provide a seamless experience for regulatory professionals, enhancing efficiency and reducing the complexity of managing submissions. **Tools/Resources Mentioned:** * Veeva Vault RIM * eCTD (Electronic Common Technical Document) format **Key Concepts:** * **Regulatory Information Management (RIM):** A system designed to manage all regulatory data, documents, and processes throughout the lifecycle of a product. * **Submission:** The package of documents and data sent to a health authority for regulatory approval or other regulatory actions. * **Regulatory Objective:** A specific goal or requirement that needs to be achieved through a regulatory submission. * **eCTD (Electronic Common Technical Document):** An internationally agreed-upon standard for organizing regulatory submissions electronically, consisting of five modules. * **Content Plan:** A structured list of all documents and information required for a regulatory submission, often organized according to the eCTD format. * **Document Type:** Predefined categories for documents within Veeva Vault RIM (e.g., clinical, non-clinical, quality) that help in organizing and classifying content for submissions. * **Lifecycle and Workflow:** The sequence of states and automated actions that a submission or document progresses through from creation to final approval and archiving, ensuring compliance and process adherence.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Oct 15, 2021
This video provides an in-depth exploration of Regulatory Information Management Systems (RIMS), their evolving definition, key drivers for implementation, and their strategic importance beyond mere compliance within the pharmaceutical and life sciences industries. Featuring insights from Lidia Canovas of Asphalion and Esteve Clark of Ennov, a RIMS vendor, the discussion highlights how RIMS are becoming increasingly critical due to the approaching implementation of ISO IDMP and other structured content submission initiatives. The conversation establishes RIMS as a foundational element for managing the complexities of global regulatory affairs, emphasizing their role in consolidating information, tracking registrations, and influencing business strategy. The discussion traces the evolution of RIMS from simple tools designed to replace spreadsheets and bespoke databases to sophisticated platforms that emulate the entire regulatory business process. It positions RIMS as the third pillar alongside document management and publishing tools, forming a cohesive regulatory ecosystem essential for handling the unified submission of structured data and documents to health authorities. A significant portion of the talk focuses on the primary motivators for companies to adopt or refresh RIMS, including the mandate for structured content submissions like IDMP and UPD, the need to modernize outdated regulatory infrastructures, and the growing recognition of RIMS as a valuable source of intellectual property and business intelligence. Furthermore, the video delves into the practical benefits of RIMS, particularly in improving impact assessment. It illustrates how RIMS can provide internal regulatory intelligence, aid in strategic decision-making regarding product portfolios, and, most notably, offer a comprehensive view of the end-to-end costs and timelines associated with organizational changes, such as manufacturing improvements. A compelling case study reveals how one company leveraged RIMS data to determine that nearly 60% of manufacturing changes had a negative financial impact after considering all factors, including regulatory timelines. The speakers also broaden the scope of RIMS applicability, asserting their utility across human medicine, veterinary products, medical devices, and API manufacturing, and stress the importance of seamless integration with other enterprise systems like Quality Management Systems (QMS) for optimal efficiency and data consistency. Key Takeaways: * **Core Function of RIMS:** Regulatory Information Management Systems are designed to consolidate all regulatory information into a single source, enabling comprehensive tracking and management of global product registrations. This replaces fragmented data stored in spreadsheets or bespoke departmental databases. * **IDMP and Structured Content as Key Drivers:** The impending implementation of ISO IDMP (Identification of Medicinal Products) and other structured content submission initiatives like UPD (Union Product Database) are the most significant catalysts for companies to adopt or upgrade their RIMS, necessitating a unified approach to data and document submission. * **RIMS as a Regulatory Ecosystem Component:** RIMS are intrinsically linked with document management and publishing tools, forming a unified "regulatory ecosystem" where structured data and documents are managed in unison to ensure consistency and compliance with evolving regulations. * **Strategic Value Beyond Compliance:** While RIMS serve as essential compliance tools, their value extends significantly to operational efficiency, strategic planning, and providing critical business intelligence. They help influence future business and regulatory strategies by offering insights into past precedents and current statuses. * **Enhanced Impact Assessment Capabilities:** RIMS significantly improve impact assessment by providing a clear view of regulatory process mechanics, allowing companies to assess internal regulatory intelligence, evaluate product performance (e.g., sunset clauses), and contribute to activities like product transfers or mergers and acquisitions. * **Data-Driven Decision Making:** A powerful application of RIMS is the ability to integrate its data with other organizational data sources (e.g., manufacturing, quality) to perform comprehensive cost and timeline analyses for changes. One example cited a company using RIMS data to discover that 60% of manufacturing improvements had a negative financial impact when all end-to-end costs and approval timelines were considered. * **Importance of System Integration:** Direct integration between RIMS and other enterprise systems, particularly Quality Management Systems (QMS), is highly advantageous. This seamless communication streamlines information flow, reduces manual effort, and ensures consistency across critical organizational functions. * **Broad Applicability Across Sectors:** RIMS are not exclusive to human medicines but are equally crucial for veterinary products, consumer health, medical devices, and even API manufacturers. The complexity of global registrations necessitates these tools regardless of the product type. * **Company Size is No Longer a Barrier:** The need for a RIMS is driven by the inherent complexity of global registrations, not company size or revenue. Small, innovative companies preparing for their first registration can benefit as much as large multinationals, and the competitive RIMS marketplace offers solutions for all scales. * **Diverse Stakeholders and Data Consumption:** While regulatory departments typically own RIMS, the data collected within them is valuable to a wide array of internal stakeholders, including clinical management, artwork and labeling, pharmacovigilance, quality management, portfolio management, intellectual property, sales, and marketing. * **Criticality of Data Exchange Capabilities:** Modern RIMS must offer robust capabilities for exposing and exchanging data with other systems, often through data marts. This ensures that valuable regulatory information is accessible and consumable by various groups across the organization, provided the context of the data is understood. * **Role of Implementation Partners:** Engaging world-class implementation partners is crucial for a successful RIMS deployment. These partners bring specialized expertise, save time and money, help navigate the RIMS landscape, and ensure the chosen solution aligns with specific company needs and integrates smoothly with existing systems. * **Proactive Adaptation to Evolving Regulations:** Adapting to new standards like ISO IDMP requires early involvement, a clear understanding of overall objectives, and proactive development. Strategic partnerships with software providers and implementation vendors are essential to ensure the RIMS continuously evolves with regulatory changes. **Examples/Case Studies:** * **Manufacturing Change Assessment:** A high-tech company utilized its existing RIMS data to analyze the economic and practical sense of manufacturing changes. This analysis revealed that almost 60% of manufacturing improvements, despite being beneficial to the manufacturing process, had a negative financial impact overall due to end-to-end costs (including regulatory affairs) and lengthy approval timelines. This led to a significant shift in how manufacturing changes were evaluated.