Videos
Browse videos by topic
All Videos
Showing 289-307 of 307 videos

Veeva Vault PromoMats Online Training: The Comprehensive Guideline | Proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva Vault PromoMats, positioning it as an indispensable, cloud-based content management solution tailored for the life sciences industry. It details how the platform streamlines the entire lifecycle of promotional materials, from collaborative creation and Medical, Legal, and Regulatory (MLR) review to multichannel distribution and electronic withdrawal of outdated content. A central theme is the platform's role in ensuring strict regulatory compliance, enhancing workflow efficiency, and fostering seamless collaboration among cross-functional teams in pharmaceutical, biotech, and medical device companies. The training outline covers core functionalities such as document creation, workflow automation, version control, digital asset management, and integration with other systems like CRM, all designed to reduce risks and accelerate time-to-market for compliant promotional content. Key Takeaways: * **Industry-Specific Content Management:** Veeva Vault PromoMats is a specialized, cutting-edge platform for managing promotional materials in the highly regulated pharmaceutical and life sciences sectors. * **Comprehensive Lifecycle Support:** It covers the full content lifecycle, including creation, MLR review, approval, distribution, tracking, and secure archiving, ensuring consistent messaging and compliance. * **Regulatory Compliance Focus:** The platform is built with compliance in mind, offering features like robust documentation, version control, audit trails, and controlled access to meet stringent regulatory requirements (e.g., FDA, EMA). * **Enhanced Efficiency and Collaboration:** PromoMats streamlines review and approval processes through automated workflows, significantly reducing manual errors, accelerating time-to-market, and improving collaboration across departments. * **Integration with Enterprise Systems:** It integrates seamlessly with other Veeva solutions and third-party systems, including CRM platforms, to ensure approved content accessibility for sales teams and efficient data exchange. * **Customization for Organizational Needs:** The platform allows for extensive customization of workspaces, templates, metadata, and review workflows to align with an organization's unique processes and branding. * **Strategic Importance and Career Opportunities:** The detailed training and discussion of career paths underscore the platform's critical role and widespread adoption within the life sciences industry, highlighting a demand for skilled professionals.

Live from Veeva Summit
Note to File: A Clinical Research Podcast
/@notetofilepodcast
Recorded live from the Veeva Summit, this video delves into critical discussions within the pharmaceutical and life sciences industries, specifically highlighting the evolving landscape of clinical research technology and stakeholder collaboration. The speakers discuss the Summit's emphasis on bringing together sponsors and sites to foster direct communication and problem-solving across the clinical trial lifecycle. A significant point of discussion is the launch of "Veeva AI," which aims to integrate data platforms with AI "agents," and the ongoing development of a full-fledged Clinical Trial Management System (CTMS). The conversation also touches upon the challenges of industry fragmentation and the need for standardization, as well as Veeva's increasing investment in solutions for clinical sites. Key Takeaways: * **Enhanced Sponsor-Site Collaboration:** The Veeva Summit actively promoted direct dialogue and collaboration between clinical trial sponsors and sites, identifying a shared understanding of challenges and a mutual desire to improve operational efficiencies throughout the protocol lifecycle. * **CROs as a Critical Missing Voice:** A notable observation was the underrepresentation of Contract Research Organizations (CROs) in these crucial sponsor-site discussions, suggesting a potential gap in comprehensive problem-solving that requires all key stakeholders at the table. * **Veeva's Strategic AI and CTMS Expansion:** Veeva announced the launch of "Veeva AI," signaling a paradigm shift towards integrating AI agents and data platforms, and is actively developing a comprehensive CTMS, indicating a significant push into AI-driven solutions and end-to-end clinical operations management. * **Industry Fragmentation and Standardization Challenges:** The speakers highlighted the pervasive issue of siloed operations and fragmentation within the clinical research ecosystem, even within sponsor organizations, underscoring the difficulty but necessity of establishing industry standards for increased productivity. * **Growing Investment in Site Solutions:** Veeva is significantly increasing its focus and investment in clinical sites, evidenced by a dedicated "site zone" at the Summit and a growing team, moving towards more holistic solutions beyond its initial electronic Investigator Site File (EISF) offering. * **Strategic Adoption of New Technologies:** For clinical sites, the adoption of new platforms like Veeva's evolving CTMS requires careful consideration of current capabilities and future roadmaps. It emphasizes the importance of providing feedback to influence product development and adopting solutions when they fully align with specific operational needs, such as integrated eSource.

Veeva Vault RIM: Comprehensive Online Training learn from experts with proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of a training program for Veeva Vault RIM, detailing its extensive functionalities for Regulatory Information Management within the pharmaceutical and life sciences industries. It covers the platform's role in streamlining compliance processes, managing regulatory documents and submissions (including IND, NDA, MAA), and ensuring regulatory success. The training curriculum explores key areas such as system configuration, user access control, document versioning, workflow automation, health authority communication, regulatory reporting, global alignment, and robust data integrity and security measures essential for compliance with standards like GxP and 21 CFR Part 11. The content underscores the platform's ability to enhance efficiency, collaboration, and reduce compliance risks across various regulatory activities Key Takeaways: * **End-to-End Regulatory Lifecycle Management:** Veeva Vault RIM offers comprehensive capabilities for managing the entire regulatory information lifecycle, from initial document authoring and submission planning to dossier management, publishing, and long-term archiving, which is crucial for pharmaceutical and life sciences companies. * **Enhanced Collaboration and Data Integration:** Veeva Vault RIM facilitates cross-functional collaboration, enabling efficient information sharing and integration of regulatory data with other critical departments such as Quality Assurance and Clinical Operations, streamlining overall operational workflows. * **Strategic Reporting and Global Alignment:** The system provides powerful reporting and analytics tools for tracking submission status, compliance trends, and key performance indicators. It also supports global regulatory alignment, helping organizations manage submissions and adhere to diverse international regulatory standards. * **Risk Management and Continuous Improvement:** Veeva Vault RIM supports proactive regulatory risk management through impact analysis for changes and fosters continuous improvement by identifying and addressing process inefficiencies, contributing to sustained regulatory excellence.

Capgemini Interview Experience | Veeva Vault Interview Questions 2025 | Capgemini Veeva Interview
The Corporate Guys
/@TheCorporateGuys
This video provides a detailed account of a Capgemini interview experience for a Veeva Vault Developer role, offering a comprehensive look into the technical and operational knowledge expected in this domain. The discussion covers a wide array of Veeva Vault functionalities, from security configurations and data management to workflow changes and regulatory information management (RIM). It also delves into support-related activities, system administration, and the importance of understanding Veeva's release cycles and compliance requirements.ai, which specializes in Veeva CRM consulting and AI solutions for the pharmaceutical and life sciences industries, understanding the technical depth and operational challenges associated with Veeva Vault is directly applicable to their service offerings, talent acquisition, and client solution strategies. Key Takeaways: * **Comprehensive Veeva Vault Expertise:** The interview questions demonstrate the need for a deep and broad understanding of Veeva Vault functionalities, encompassing security models (Atomic, DAC), data loaders, workflow configuration, and specific product modules like Regulatory Information Management (RIM). * **Strong Operational & Support Acumen:** A significant focus on daily activities, incident management, issue resolution processes (L1, L2, L3 support), and handling critical situations like system outages highlights the importance of operational excellence and robust support capabilities for Veeva Vault professionals. * **Regulatory Compliance Integration:** Questions concerning GxP applications, IQ OQ, and the nuances of RIM data (transactional, global, master) underscore Veeva Vault's critical role in maintaining regulatory adherence within the pharmaceutical and life sciences sectors. * **Veeva Product Lifecycle & Release Management:** The emphasis on Veeva General Releases, key features from recent updates (e.g., 24r1, 24r2), and processes for major releases indicates the necessity for professionals to stay current with Veeva's evolving platform and manage its deployment effectively. * **Advanced System Administration:** Interview topics covering user access management across multiple environments, Vault environment refresh procedures, and pre-release availability demonstrate the demand for skilled system administrators capable of complex Veeva Vault environment control.

Veeva Vault online training learn and improve your skill and Knowledge With Experts trainer
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva Vault, positioning it as a critical cloud-based content and data management solution specifically designed for the life sciences industry. The training covers fundamental aspects such as understanding Veeva Vault's purpose in streamlining processes, enhancing collaboration, and maintaining compliance. It delves into practical skills like navigating the platform, setting up user profiles and permissions, and mastering document management fundamentals including uploading, version control, and organizing files. The course also explores advanced functionalities such as collaborative workflows, customizing metadata, implementing robust data security and access controls, and utilizing advanced search capabilities. A significant portion is dedicated to regulatory compliance, electronic signatures, audit trails, workflow automation, and integration with other enterprise systems. The video further highlights Veeva Vault's applications in quality management, clinical trials, and regulatory submissions, underscoring its role in ensuring data integrity and adherence to industry standards. Key Takeaways: * **Veeva Vault as a Core Life Sciences Platform:** The video reinforces Veeva Vault's essential role as a document and data management system specifically designed for the life sciences, critical for managing regulated content across clinical, regulatory, and quality operations. * **Comprehensive Compliance & Data Integrity Features:** It details Veeva Vault's built-in capabilities for regulatory adherence, including electronic signatures, audit trails, version control, and robust access controls. * **Workflow Automation & Collaboration:** The platform's emphasis on configurable workflows, task assignment, and notification systems highlights its ability to automate routine tasks, streamline document review and approval processes, and enhance cross-functional collaboration.ai to integrate AI and LLM solutions for intelligent automation within these workflows. * **Data Engineering & Integration Potential:** The discussion on customizing metadata, advanced search, reporting/analytics, and API integration points to Veeva Vault's potential as a central hub for data.ai targets, such as clinical operations, medical affairs, and regulatory compliance.ai aims to solve.

Important Veeva Interview Questions | Veeva Vault Interview Questions 2025 | The Corporate Guys
The Corporate Guys
/@TheCorporateGuys
This video, presented by Vaibhav Agrawal from The Corporate Guys, delves into 15 important and recently asked interview questions pertaining to Veeva Vault. The discussion covers a broad spectrum of Veeva Vault functionalities, including how new general releases (like 24R3) are tested and assessed, the mechanics of Crosslink, and the critical role of Dynamic Access Control (DAC). The speaker also explains practical aspects such as retrieving deleted documents, various methods for extracting document and object metadata, and the utility of Flash Reports. Furthermore, the video touches upon fundamental Veeva concepts like Entry Criteria, Entry Actions, User Actions, different ways to create documents, types of field dependencies, Application Roles, and the distinctions between Document and Object Life Cycles. The content is geared towards professionals seeking to deepen their understanding of Veeva Vault and prepare for interviews in the life sciences sector, providing insights into both technical configurations and operational best practices. Key Takeaways: * **Veeva Release Management:** Understanding the process of testing and assessing new Veeva general releases (e.g., 24R3 features, auto-on vs. configurable features) is crucial for maintaining system integrity and compliance in a regulated environment. * **Core Veeva Functionalities:** Key concepts like Crosslink (for document creation and field value copying), Dynamic Access Control (DAC) for granular permissions, and User Role Setup are fundamental to Veeva Vault administration and security. * **Data Management & Retrieval:** Practical knowledge of retrieving deleted documents (within 30 days via Veeva support and API) and various methods for extracting metadata (Loader, UI export, API) is essential for operational efficiency and audit readiness. * **Reporting & Automation:** Flash Reports (scheduled reports) and their evolving capabilities (e.g., custom text in 24R3) offer powerful tools for automated communication and business intelligence within Veeva. * **Workflow & Configuration Basics:** A solid grasp of Entry Criteria, Entry Actions, User Actions, different document creation methods, and the three types of Field Dependencies (Field-based, Document Type-based, Document Life Cycle-based) is vital for effective Veeva Vault configuration and workflow design. * **Life Cycle Management:** Differentiating between Document and Object Life Cycles is a core aspect of managing content and data within Veeva Vault, directly impacting workflows and regulatory compliance.

Veeva Vault Interview Questions | Compliance Group Interview Experience | Veeva Interview Experience
The Corporate Guys
/@TheCorporateGuys
This video details a job interview experience for a Veeva Vault developer position at a company named "Compliance Group." The transcript outlines the multi-round recruitment process, focusing heavily on the technical interview questions for a candidate with six years of dedicated Veeva Vault experience. Key areas of questioning included Veeva Vault's security model, job scheduling and automation, general releases, deployment and migration best practices (including data packages and Vault Loaders), impact assessment, field-level security, workflows, life cycles, and object configuration. The managerial and HR rounds focused on career motivations and fit within a smaller, growing organization. Key Takeaways: * **Comprehensive Veeva Vault Technical Expertise:** The interview process demands in-depth knowledge of Veeva Vault's core functionalities, including security profiles, permission sets, user roles, job scheduling, automation tool integration, general releases, and intricate deployment and migration strategies. * **Criticality of Compliance and Best Practices:** The focus on "Compliance Group" and detailed questions about deployment considerations, migration impact assessments, and data integrity underscore the paramount importance of regulatory compliance and adherence to industry best practices in Veeva Vault implementations. * **Business Value Articulation:** Candidates are expected to not only possess technical skills but also to articulate the business use cases and benefits of the solutions they implement, such as automation tools integrated with Veeva Vault. * **Strategic Specialization in Regulated Tech:** The interviewee's career path highlights the value of deep, specialized experience in niche enterprise technologies like Veeva Vault within regulated industries, demonstrating a strategic move towards a compliance-focused role. * **Holistic Interview Preparation:** Beyond technical proficiency, success in such roles requires preparation for managerial questions concerning career motivations, organizational fit, and the ability to address challenges and lead teams, even in highly technical positions.

Veeva Vault Quality: The Complete Guide from experts with Proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva Vault Quality, a cloud-based quality management system vital for highly regulated industries such as Pharmaceuticals and Life Sciences. It emphasizes how the platform streamlines quality processes, ensures regulatory compliance (including FDA, GxP, and ISO standards), and enhances operational efficiency. The content, framed as an online training course, delves into Veeva Vault Quality's architecture, core functionalities like document management, change control, risk management, and audit management, as well as its integration capabilities with other enterprise systems like ERPs. The discussion also highlights the benefits of flexible, tailored online training for professionals and briefly acknowledges the transformative role of technological innovations such as AI and IoT in modern quality management practices. Key Takeaways: * Veeva Vault Quality is presented as an essential, comprehensive cloud-based Quality Management System (QMS) specifically designed for the life sciences industry, addressing critical needs in pharmaceuticals, biotech, and medical devices. * The system offers robust functionalities for managing core quality processes, including document control, change control, risk assessment and mitigation, audit planning and tracking, and handling deviations and complaints. * Veeva Vault Quality supports integration with other enterprise systems, such as ERPs, to facilitate seamless data exchange and ensure data integrity across an organization's operational landscape. * The video briefly but significantly notes that technological innovations like Artificial Intelligence (AI) and the Internet of Things (IoT) are transforming quality management practices, indicating a future direction where AI solutions can augment platforms like Veeva Vault Quality. * The platform provides end-to-end visibility into quality processes, enabling data-driven decision-making, streamlining workflows, and fostering collaboration among cross-functional teams.

Veeva System Case Study || Veeva System Migration Specialist Interview Case Study || Veeva Vault Que
The Corporate Guys
/@TheCorporateGuys
This video presents a detailed case study for a Veeva Systems Migration Specialist interview, focusing on the practical execution of data and document migration to Veeva Vault Quality. The speaker, Vaibhav Agrawal, outlines a comprehensive migration strategy, including source data analysis, mapping document types, subtypes, classifications, and associated life cycles, as well as defining metadata fields and their dependencies. A significant portion of the discussion is dedicated to managing complex document versioning, particularly how to identify and migrate main versions while preserving historical versions within the Veeva Vault structure, often targeting a "Steady State Effective or Approved" status. The presentation also covers object creation with parent-child relationships using Veeva loader sheets and the importance of structuring a technical solution as a client-facing presentation. Key Takeaways: * **Veeva Vault Migration Process:** The video details a structured approach to Veeva Vault data migration, covering source data analysis, mapping strategies for document types and metadata, CSV preparation, and systematic data loading with error rectification. * **Complex Document Versioning:** A critical insight is the method for handling document versioning in Veeva Vault, which involves identifying main versions (e.g., major version >=1, minor version = 0) for initial upload, extracting document IDs, and then using these IDs to link and preserve minor versions within the version history tree. * **Object Relationship Management:** The case study highlights the importance of correctly identifying and establishing parent-child relationships between objects (e.g., Category and Sub-category) during migration, ensuring data integrity within Veeva. * **Veeva Loader Sheets & API/VQL:** The reliance on Veeva loader sheets for bulk data and document creation/update is a core technical takeaway, with a mention of API/VQL for advanced functionalities or data retrieval. * **Client-Centric Technical Presentation:** The speaker's advice to frame a technical interview case study as a client presentation offers a valuable perspective on how to communicate complex solutions effectively, focusing on process, strategy, and outcomes. * **Industry-Specific Context:** The discussion implicitly underscores the regulatory and operational rigor required in the pharmaceutical and life sciences industries, where precise document control, versioning, and data integrity within systems like Veeva Vault Quality are paramount.
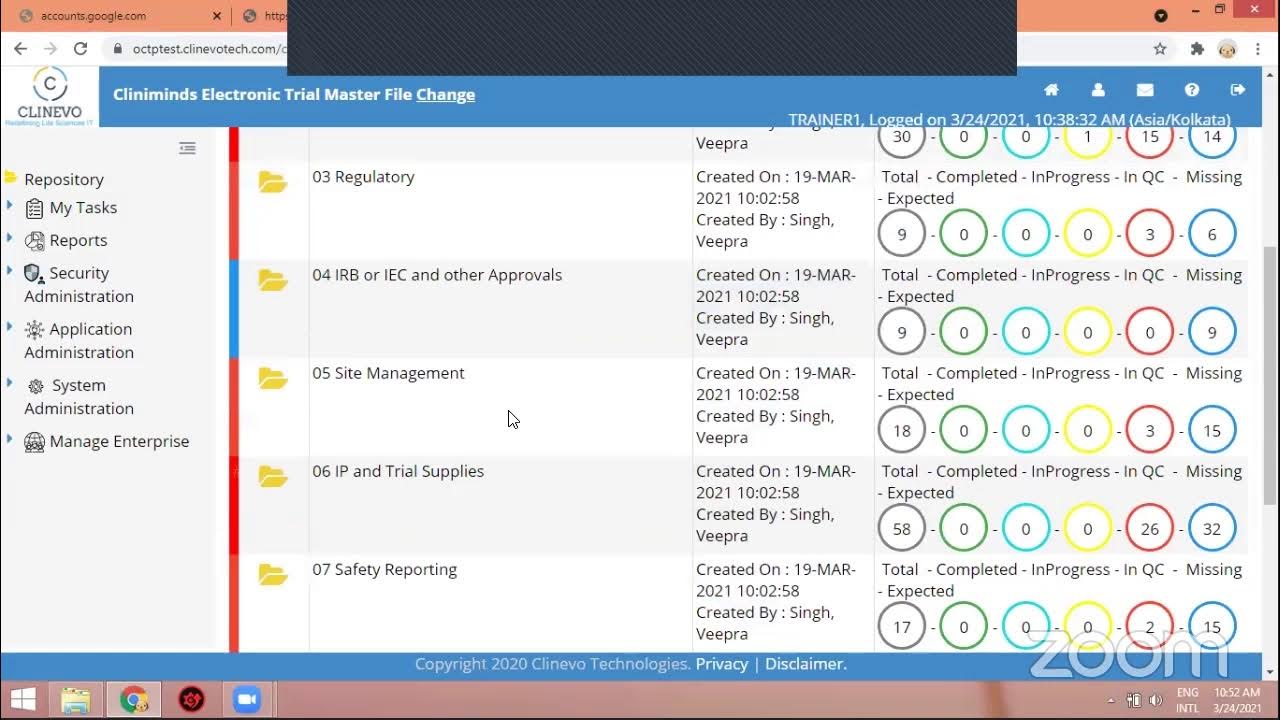
eTMF Software Hands on Practice - Cliniminds || Electronic Trial Master File
Cliniminds India
/@ClinimindsIndia
This video provides a hands-on, practical demonstration of utilizing an Electronic Trial Master File (eTMF) software system, focusing specifically on the critical workflow processes involved in managing clinical trial documentation. The primary purpose is to guide users through the step-by-step procedures for uploading, describing, and progressing essential documents like study protocols and case report forms (CRFs) through a regulated approval cycle. The tutorial emphasizes the importance of structured document management within a clinical research context, highlighting how an eTMF system facilitates organization, version control, and compliance. The demonstration begins with the fundamental action of uploading a document into a designated folder, illustrating how to use placeholders and context menus to initiate the upload process. It then delves into the crucial metadata associated with each document, such as providing a description, assigning a name, and setting workflow parameters like "auto-publish," along with effective and expiry dates. A significant portion of the video focuses on the document lifecycle, transitioning from an initial "open" status to subsequent stages like "pending for review" and "approved." This progression is managed through specific actions like "check-in," which is vital for version control and moving documents through the predefined workflow. Throughout the session, the instructor guides a participant through the software interface, troubleshooting minor operational issues like ensuring a document is selected before attempting a right-click action or saving a document before assigning reviewers. The core methodology demonstrated revolves around a role-based workflow, where documents move from an author to a reviewer and then an approver. This structured approach ensures that all necessary checks and balances are in place, aligning with regulatory requirements for clinical trial documentation. The practical examples of uploading a "protocol" and a "sample case report form" underscore the system's application to diverse clinical trial artifacts, reinforcing the necessity of meticulous documentation for regulatory adherence and audit readiness. Key Takeaways: * **eTMF as a Centralized Repository:** The video demonstrates an eTMF system as a crucial digital platform for organizing and managing all essential documents related to a clinical trial, ensuring a single source of truth for regulatory compliance and operational efficiency. * **Structured Document Upload Process:** Users are guided on how to upload documents using specific interface elements like placeholders, right-click menus, and "add document" options, emphasizing the systematic approach required within an eTMF. * **Importance of Document Metadata:** Critical information such as protocol description, document name, effective date, and expiry date must be accurately entered during upload. This metadata is essential for searchability, categorization, and compliance tracking. * **Automated Workflow Configuration:** The eTMF system allows for configuring workflows, such as "auto-publish," which dictates how documents progress through their lifecycle, minimizing manual intervention for routine tasks. * **Role-Based Document Review and Approval:** Clinical trial documents undergo a rigorous review and approval process involving different roles (e.g., author, reviewer, approver). The system facilitates assigning these roles to specific individuals to ensure accountability and compliance. * **Document Status Tracking:** The eTMF clearly displays the current status of each document (e.g., "open," "pending for review," "approved"), providing transparency and enabling real-time monitoring of documentation progress. * **"Check-in" for Version Control and Workflow Progression:** The "check-in" functionality is critical for formalizing changes, creating new versions, and moving a document to the next stage in its predefined workflow (e.g., from editing to review). * **Iterative Document Management:** The process of uploading, saving, reviewing, editing, and approving documents is iterative, often requiring multiple steps and interactions within the eTMF system to reach final approval. * **Handling Diverse Clinical Documents:** The demonstration covers the uploading of different types of essential clinical trial documents, specifically a "protocol" and a "case report form (CRF)," highlighting the system's versatility for various trial artifacts. * **Troubleshooting Common User Errors:** The tutorial implicitly addresses common user pitfalls, such as the necessity to first select a document before performing actions like right-clicking or ensuring a document is saved before attempting to assign reviewers. * **Regulatory Compliance Foundation:** The structured workflows, role assignments, and audit trails inherent in an eTMF system are foundational for meeting regulatory requirements from bodies like the FDA and EMA, ensuring GxP and 21 CFR Part 11 adherence. Key Concepts: * **eTMF (Electronic Trial Master File):** A digital system used to manage and store all essential documents for a clinical trial in a compliant and organized manner. * **Protocol:** A document that describes the objectives, design, methodology, statistical considerations, and organization of a clinical trial. * **Case Report Form (CRF):** A document, paper or electronic, designed to record all protocol-required information to be reported to the sponsor on each trial subject. * **Workflow:** A sequence of tasks or processes through which a document passes from initiation to completion, often involving multiple users and stages like review and approval. * **Auto-Publish:** A workflow setting that automatically publishes a document or moves it to the next stage upon meeting certain conditions, without manual intervention. * **Reviewer/Approver:** Roles assigned to individuals responsible for examining and formally sanctioning documents within the eTMF workflow. * **Check-in:** The action of saving a document back into the eTMF system, often creating a new version and potentially triggering the next step in its workflow. * **Document Status:** The current state of a document within its lifecycle (e.g., open, pending for review, approved, archived). Tools/Resources Mentioned: * An unspecified eTMF software system is demonstrated for hands-on practice.

Lunch and Learn with Veeva
Black Women In Clinical Research
/@BlackWomenInClinicalResearch
This video provides an in-depth exploration of Veeva Systems, a prominent cloud-based software, data, and services provider for the life sciences industry. Hosted by Danielle Mitchell of Black Women In Clinical Research, the session features Eric Severiano from Veeva, who offers a comprehensive overview of the company's mission, values, product offerings, growth trajectory, and career opportunities. The primary purpose is to educate the audience, particularly clinical research professionals, about Veeva's ecosystem and potential career paths within the organization, emphasizing its commitment to diversity and employee success. Eric Severiano details Veeva's extensive involvement across the entire life sciences product lifecycle, from initial research and clinical trials (Development Cloud) through regulatory approval and commercialization (Commercial Cloud). He highlights Veeva's growth from a small startup to a global company with over 6,700 employees, aiming for 10,000 by 2025. A significant portion of the discussion is dedicated to Veeva's core values—doing the right thing, customer success, employee success, and speed—which are not just aspirational but legally enshrined through its status as a Public Benefit Corporation (PBC). This PBC status underscores Veeva's commitment to broader stakeholders, including customers, employees, and communities, beyond just shareholders. The presentation also delves into Veeva's progressive workplace policies, such as its permanent "work anywhere" model, which predates the pandemic for many roles and now extends to all employees. Career development is a key theme, with a focus on "Generation Veeva" programs designed for early-career professionals in various disciplines like engineering, consulting, analytics, and sales. Furthermore, Eric outlines Veeva's robust Diversity, Equity, and Inclusion (DEI) initiatives, which concentrate on talent attraction, fostering an inclusive culture, and providing education and awareness through internal trainings and employee resource groups. The latter part of the session includes a Q&A segment addressing specific roles, the interview process, and Veeva's expanding business scope, including its data offerings, management consulting services, and the Quality Management System (QMS) Vault, which is now used by non-life sciences companies. Key Takeaways: * **Veeva's Comprehensive Life Sciences Support:** Veeva Systems provides cloud-based software, data, and consulting services that span the entire life sciences product lifecycle, from research and development (Development Cloud) to commercialization and sales (Commercial Cloud). * **Dual Cloud Offerings:** The Development Cloud supports pre-FDA approval activities like research, clinical trials, and regulatory processes, while the Commercial Cloud handles post-approval aspects such as marketing, sales interactions, and related data. * **Veeva Vault Ecosystem:** Veeva offers specialized "Vaults" for various functions, including Clinical Data, Clinical Operations, Regulatory, Quality, and Drug Safety. These platforms are designed to be user-friendly, cloud-based, and easily configurable, aiming to standardize processes and facilitate decentralized trials. * **Core Company Values:** Veeva's operations are guided by four key values: "do the right thing," customer success, employee success, and speed, which are frequently discussed and integrated into decision-making. * **Public Benefit Corporation (PBC) Status:** In 2020, Veeva became a PBC, legally obligating the company to consider the interests of all stakeholders—customers, employees, and communities—beyond just maximizing shareholder profit, a unique commitment for a public company of its size. * **Permanent "Work Anywhere" Policy:** Veeva has a long-standing and permanent policy allowing employees to work from anywhere, fostering flexibility and productivity, a model that was expanded to all roles in 2020. * **Generation Veeva Development Programs:** The company offers structured, full-time development programs for early-career professionals in engineering, consulting, business consulting, analytics, and sales, serving as a pipeline for future leadership and industry talent. * **Robust DEI Initiatives:** Veeva's DEI efforts focus on challenging talent pipeline myths through targeted attraction strategies, ensuring equitable evaluation processes, fostering an inclusive culture, and providing education on topics like bias and microaggressions, supported by employee resource groups. * **Career Opportunities for Clinical Research Professionals:** Individuals with clinical research backgrounds, such as CRAs, have a significant advantage due to their industry knowledge. Veeva offers diverse roles within clinical-specific teams, including product management, sales, engineering, consulting, quality assurance, and marketing. * **Consultant vs. Strategy Roles:** Implementation and strategic guidance on platform usage typically fall under "consultant" or "Professional Services" roles at Veeva. "Strategy roles" are generally distinct, focusing on broader market vision, product positioning, and pricing. * **Veeva QMS Expansion:** Veeva's Quality Management System (QMS) Vault, designed for managing compliant documentation and processes, is expanding its reach beyond life sciences to industries like cosmetics and manufacturing. * **Business Growth Areas:** Veeva is actively expanding its data offerings (e.g., patient, prescription data), growing its management consulting services (e.g., optimizing field teams), and extending its QMS solutions to non-life sciences sectors, creating new job opportunities. * **Comprehensive Interview Process:** The typical interview process involves 3-4 stages, including a recruiter screen, conversations with potential managers, a deep dive into work history, a hands-on exercise relevant to the role, and a discussion focused on culture fit and personal motivations. * **Emphasis on Internal Mobility:** Veeva strongly encourages and supports internal career growth, with many employees moving between different roles and departments as the organization expands. * **Potential for External Veeva Certification:** There is ongoing internal discussion about developing external training and certification programs for Veeva products, which could significantly benefit industry professionals seeking to enhance their skills and marketability. **Tools/Resources Mentioned:** * Veeva CRM * Veeva Vault (general platform) * Veeva Development Cloud * Veeva Commercial Cloud * Veeva Clinical Data Vault * Veeva Clinical Operations Vault * Veeva Regulatory Vault * Veeva Quality Vault * Veeva Drug Safety Vault * Veeva EDC Vault (Electronic Data Capture) * Veeva QMS (Quality Management System) **Key Concepts:** * **Public Benefit Corporation (PBC):** A legal designation for a for-profit corporation that commits to operating in a manner that benefits society and the environment, in addition to its shareholders. * **Industry Cloud:** A specialized cloud platform tailored to the unique needs and regulatory requirements of a specific industry, in this case, life sciences. * **Decentralized Trials:** Clinical trials conducted with less reliance on physical sites, often leveraging technology to allow participants to engage remotely, improving accessibility and efficiency. * **Generation Veeva:** Veeva's suite of development programs designed to train and integrate early-career professionals into various functional areas of the company. * **Work Anywhere Policy:** A company policy that allows employees to perform their job duties from any location, promoting flexibility and work-life balance. **Examples/Case Studies:** * **COVID-19 Response:** Veeva provided its remote pharmaceutical rep software for free for a year during the pandemic lockdown, demonstrating its "do the right thing" value by supporting customers when traditional in-person interactions were impossible. * **Data Offering Expansion:** Veeva acquired an organization approximately three years prior to the video to significantly expand its data offerings, including patient and prescription data, highlighting its growth strategy in this area.

TQA Cloud QMS Bootcamp | Implementing a Quality Management System | Session 3 | #QualityMatters
Texas Quality Assurance | #QualityMatters Podcast
/@texasqa
This video provides an in-depth exploration of implementing a Quality Management System (QMS), focusing specifically on process mapping and an integrative process approach. Kyle Chambers, CEO and founder of Texas Quality Assurance, presents this session as part of a condensed, free online QMS bootcamp, originally a four-day course. The core purpose is to equip leadership and quality management teams with the skills to develop, implement, and maintain an effective QMS, emphasizing the critical role of quality management and assurance programs for business success, particularly for small businesses. The session delves deeply into the "process approach," highlighting its importance as a systematic way to view the interconnected pieces of a management system, moving beyond superficial document control to understand how various elements interact. A central framework discussed is the Plan-Do-Check-Act (PDCA) cycle, presented as a fundamental and iterative model essential at every step of process mapping. Chambers stresses that the cycle begins with a clear "quality policy" (broadly defined to include health, safety, or environmental policies), which must align with the organization's values and mission, serving as more than just lip service for compliance. He also notes that Edward Deming's original model used "Study" instead of "Check," underscoring the depth of analysis required. The discussion progresses to the practical application of the PDCA cycle, detailing each phase: Plan (defining desired outcomes, goals, metrics, KPIs), Do (performing the work, utilizing work instructions), Check (pre-defined measures against planning requirements, inspections), and Act (taking actions like accepting, reworking, or scrapping, leading to continuous improvement or corrective/preventative actions). Chambers critiques the standard ISO 9001 diagram for its complexity, advocating for a more intuitive "integrative process approach" model. This model emphasizes identifying resources/inputs, goals/objectives, expected outputs, and checks/measures, with the "process" and "controls" fitting in the middle. He introduces "integrative thinking" as the ability to constructively resolve tensions between opposing models by creating a new, superior solution that incorporates elements of both, which is core to the process approach's method-agnostic nature. Key Takeaways: * **QMS Bootcamp Value:** The QMS Bootcamp is a valuable, condensed online course designed to teach leadership and quality management teams how to develop, implement, and maintain a QMS, offered freely to support businesses. * **The Process Approach is Fundamental:** A systematic view of interconnected processes is crucial for understanding how a management system truly functions, moving beyond simple document control. * **PDCA Cycle is Iterative:** The Plan-Do-Check-Act (PDCA) cycle is not a one-time concept but an integral, iterative framework that must be applied at every stage of process mapping and QMS management. * **Quality Policy Alignment:** A "quality policy" (which can encompass health, safety, or environmental aspects) must be in strong alignment with the organization's values and mission to drive long-term success, not just for compliance. * **"Study" Emphasizes Depth:** Edward Deming's original "Plan-Do-Study-Act" cycle highlights the importance of deeply studying processes rather than merely checking them, advocating for thorough analysis. * **Focus on Added Value:** Every process step should be continually questioned for its added value to the organization; steps that cannot justify their existence are potential waste to be eliminated. * **Factual-Based Decision Making:** Improvement of processes must be based on data and information, aligning with the quality management principle of evidence-based decision making, rather than subjective opinions. * **Define Desired Outcomes First:** The single most critical part of the planning process is defining desired outcomes, purpose, and scope; without these, efforts are likely wasted. * **Distinguish Process Procedures from Work Instructions:** Understand the difference between a specific, single-function work instruction (e.g., lockout/tagout) and a broader process procedure that considers interconnected processes and their wider impact. * **Pre-defined Checks are Essential:** The "Check" phase of PDCA should involve pre-defined measures, acceptance criteria, and customer requirements to ensure consistency and effectiveness in evaluating work. * **The "Act" Phase Drives Improvement:** The "Act" phase is where decisions are made (accept, rework, scrap), generating valuable data for corrective and preventative actions, feeding into continuous improvement. * **Integrative Thinking Resolves Conflict:** Applying "integrative thinking" helps resolve disagreements by focusing on problem requirements and combining elements from opposing solutions to create a superior, new model. * **Practical Process Mapping Model:** A more effective process map identifies resources/inputs, goals/objectives, expected outputs, and checks/measures, allowing the "process" and "controls" to be flexibly defined in between. * **Process Maps as Internal Tools:** Process maps should be treated as evergreen, internal guides for managers and owners, not controlled documents, to avoid unnecessary complexity in audit processes and encourage continuous editing and improvement. * **Process Maps for Conflict Resolution:** Utilizing a process map to define requirements, limits, vision of good, and checks can effectively reduce tensions and solve problems when teams or managers have differing opinions. **Tools/Resources Mentioned:** * **TQA Cloud:** Texas Quality Assurance's flagship QMS software product. * **Texas Quality Assurance Website:** Offers free samples of process procedures. * **Harvard Business Review on Audible:** Recommended for learning about integrative thinking. * **OSHA CFRs (Code of Federal Regulations):** Specifically mentioned CFR 1910 for general industry, as a critical input for health and safety management systems. * **ISO 9001:** The international standard for Quality Management Systems, referenced for its principles and diagrams. **Key Concepts:** * **Quality Management System (QMS):** A formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. * **Process Mapping:** The visual representation of the steps and decisions involved in a process, used to understand, analyze, and improve workflows. * **Process Approach:** A management strategy that views an organization as a system of interconnected processes, focusing on how these processes interact to achieve desired outcomes. * **PDCA Cycle (Plan-Do-Check-Act/Study):** An iterative four-step management method used for the control and continuous improvement of processes and products. * **Integrative Thinking:** The ability to constructively consider opposing ideas simultaneously to generate a new, superior solution that incorporates elements of both. * **Integrative Process Approach:** A methodology that combines integrative thinking with the process approach to build effective QMS by considering numerous variables (interested parties, customers, employees, competitors, capabilities, costs, industry trends, regulatory environment) into a cohesive solution. * **Quality Policy:** A formal statement by management, closely linked to the business plan and marketing approach, which defines the overall intentions and direction of an organization with regard to quality. * **Fact-based Decision Making:** A quality management principle emphasizing that effective decisions are based on the analysis of data and information. * **Work Instruction vs. Process Procedure:** A work instruction is a detailed, step-by-step guide for a specific task, often for one location or function. A process procedure describes a broader process, considering its interconnectedness and impact on other organizational functions. **Examples/Case Studies:** * **Coffee Pot Process Mapping:** A lighthearted example mentioned from another video, used to illustrate the absurdity and simplicity of initial process mapping exercises. * **Powered Industrial Trucks (Forklift) Program:** A detailed, real-world example used to demonstrate the integrative process approach. This included: * **Resources/Inputs:** OSHA CFR 1910, current operating environment (ramps, docks, trailer types), and employee experience levels. * **Goals/Objectives:** Zero accidents, zero injuries, saving time and effort, and ensuring proper maintenance to reduce costs. * **Outputs:** Zero incidents, improved efficiency, reduced costs. * **Checks/Measures:** Annual internal audits, comprehensive training programs (classroom, quiz, practical driving exam), and daily pre-operational maintenance inspections using laminated checklists on forklifts. * **Process/Controls:** Establishing a forklift in each shop, preventative maintenance schedules, and training schedules, with specific procedures for the PIT program and training.

How to become Project Assistant||Project Specialist||Clinical Trial
Vikas Singh
/@VikasSinghPharmalive
This video provides an in-depth exploration of the Project Assistant and Project Specialist roles within clinical trials, offering a comprehensive guide for individuals aspiring to enter or advance in the clinical research field. The speaker, Vikas Singh, outlines the essential qualifications, detailed responsibilities, potential career progression paths, and salary expectations associated with these positions. The primary purpose is to educate and empower viewers with practical information to pursue a career as a Project Assistant, emphasizing the critical support functions these roles provide within clinical study operations. The presentation begins by defining the Project Assistant's role as a crucial support function for Project Coordinators (PCs) and Clinical Trial Managers (CTMs), who often manage heavy workloads. It then systematically details the eligibility criteria, primarily focusing on academic backgrounds in life sciences such as B.Pharm, M.Pharm, B.Sc, M.Sc, or B.Tech in Biotechnology, coupled with essential computer literacy. The core of the video delves into the day-to-day responsibilities, which span various aspects of clinical study management, from ensuring stakeholder training compliance to meticulous documentation and data management. Throughout the video, the speaker emphasizes the practical aspects of the role, highlighting tasks such as collecting training logs, managing study files, handling the Trial Master File (TMF) and electronic Trial Master File (eTMF), and tracking clinical supplies. The discussion also covers patient enrollment management, including creating and updating patient master files, and assisting with project timelines and status report preparations. The video concludes by outlining promising career advancement opportunities, such as transitioning into roles like TMF Specialist, Clinical Research Associate (CRA), Project Manager, or Clinical Trial Manager, and provides insights into typical salary ranges for both freshers and experienced professionals in the field, often within Contract Research Organizations (CROs) like IQVIA, Syneos Health, and Parexel. Key Takeaways: * **Role Definition and Purpose:** A Project Assistant (PA) or Project Specialist provides essential support to Project Coordinators (PCs) and Clinical Trial Managers (CTMs) in clinical studies, helping to manage their significant workload and ensure smooth operational execution. * **Eligibility Criteria:** Aspiring Project Assistants typically require a background in life sciences, including degrees such as B.Pharm, M.Pharm, B.Sc, M.Sc (Life Sciences), or B.Tech (Biotechnology). Basic computer knowledge is also a fundamental prerequisite for the role. * **Core Responsibilities in Clinical Studies:** PAs are assigned specific clinical studies and are responsible for performing related activities, working under the guidance of CTMs and PCs. * **Training and Documentation Management:** A key responsibility involves ensuring that all central stakeholders involved in a clinical study have completed their required training. This includes collecting and recording signatures on training logs to maintain compliance. * **Study File and Correspondence Management:** PAs are tasked with initiating and maintaining study files, which includes converting all project-site communications into PDF format for proper documentation and record-keeping. * **Trial Master File (TMF) Management:** A significant duty is managing the Trial Master File (TMF) and electronic Trial Master File (eTMF), ensuring that all essential documents are accurately filed and maintained, which is crucial for regulatory compliance and audit readiness. * **Clinical Supply and Patient Data Management:** PAs are responsible for requesting and tracking clinical supplies for study sites. They also create and update a master file for enrolled patients, meticulously recording and updating important enrollment-related information. * **Project Tracking and Reporting:** The role involves updating project timelines and assisting with various project tracking activities, including the preparation of status reports to keep stakeholders informed of study progress. * **Career Progression Opportunities:** After gaining 1-2 years of experience as a Project Assistant, individuals become eligible for advanced roles such as TMF Specialist, Clinical Research Associate (CRA), Project Manager, or Clinical Trial Manager, demonstrating a clear career ladder within clinical research. * **Salary Expectations:** For freshers, the annual income typically ranges from 4 to 6 Lakhs INR, while experienced professionals can expect 7 to 10 Lakhs INR per annum, with additional performance-based increments. * **Major Employers:** Key employers for Project Assistant roles are Contract Research Organizations (CROs) and pharmaceutical companies, with prominent examples including IQVIA, Syneos Health, Parexel, Labcorp, ICON, and PPD. * **Continuous Learning and Resources:** The speaker encourages viewers to join a dedicated Telegram channel for daily updates related to pharma jobs and clinical research courses, highlighting the importance of continuous professional development. Tools/Resources Mentioned: * **eTMF (electronic Trial Master File):** A system for managing clinical trial documents electronically. * **Telegram Channel:** A platform for sharing updates on pharma jobs and clinical research courses. Key Concepts: * **Project Assistant (PA) / Project Specialist:** An entry-to-mid-level role providing administrative and operational support in clinical trials. * **Project Coordinator (PC):** A role responsible for coordinating various aspects of a clinical trial. * **Clinical Trial Manager (CTM):** A senior role overseeing the operational aspects of clinical trials. * **Clinical Study / Clinical Trial:** Research studies conducted on human volunteers to evaluate the safety and efficacy of new drugs, devices, or treatments. * **Trial Master File (TMF):** A collection of essential documents that individually and collectively permit the evaluation of the conduct of a clinical trial and the quality of the data produced. * **Clinical Research Associate (CRA):** A professional who monitors clinical trials at investigator sites. * **Contract Research Organization (CRO):** A service organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis.

Veeva 22R3 Release Questions and Answers || Veeva 22R3 New Features || Veeva Vault Certification
The Corporate Guys
/@TheCorporateGuys
This video provides an in-depth exploration of the new features introduced in the Veeva Vault 22R3 release, specifically tailored for individuals preparing for the Veeva Vault certification exam. The speaker, Vaibhav Agrawal, begins by outlining critical information regarding the certification process, including deadlines, the number of attempts allowed, and passing score requirements. He then systematically delves into several key new features, explaining their functionality, configuration steps, and practical implications for administrators and users within the Veeva Vault ecosystem. The presentation emphasizes how these updates address common challenges and enhance the platform's capabilities in areas such as data management, workflow automation, and reporting. The core of the video focuses on practical enhancements that improve user experience and administrative control within Veeva Vault. Each new feature is presented with a clear explanation of its purpose and how it can be configured or utilized. For instance, the discussion on "Person Object Duplicate Detection" highlights a solution to prevent redundant user accounts, detailing the configuration settings for matching rules based on various parameters like email or name. Similarly, the "Notification Email Notification Status" feature is presented as a crucial tool for troubleshooting email delivery issues, providing administrators with visibility into the success or failure of notifications. The speaker's approach is highly practical, often relating the features back to real-world scenarios and potential exam questions. Further into the presentation, the video covers more technical and administrative improvements. The "Object Reference Field Formula" feature is explained as an advancement that allows for more complex formula configurations by enabling the use of object reference fields within text or ID functions, thereby enriching reporting and automation possibilities. The introduction of "Output Package Support for Migrating Group Data" addresses a significant pain point for administrators, allowing for the automated migration of user groups between Veeva Vault environments, which previously required manual effort. The discussion culminates with features like "Limit Workflow Participants" and "Document with Object Report Type," which offer enhanced control over workflow assignments and more flexible reporting capabilities by linking documents with related business objects. The speaker concludes by reiterating key points that are likely to appear on the certification exam, reinforcing the practical and exam-oriented nature of the content. Key Takeaways: * **Veeva Vault 22R3 Certification Details:** The certification exam for the 22R3 release must be completed by April 14, 2023. Candidates are allowed two attempts; a score of 80% or higher on the first attempt qualifies, while a score above 50% but below 80% allows for a second attempt. Scoring below 50% on the first attempt disqualifies the candidate. * **Person Object Duplicate Detection:** This new feature helps prevent the creation of duplicate person records (e.g., user accounts) in Veeva Vault. Administrators can configure detection settings to check for duplicates based on parameters like first name, last name, email address, or username, with options for exact or fuzzy matching. * **Notification Email Notification Status:** A highly valuable feature for troubleshooting, it allows administrators to check the delivery status of email notifications sent to specific users or roles. This can be accessed via the "Operations" tab, providing clarity on whether a notification was successfully sent or if there were delivery issues. * **Object Reference Field Formula:** Veeva Vault 22R3 enhances formula capabilities by allowing the use of object reference fields within formulas. This means administrators can now reference related objects directly within text functions (to retrieve names) or ID functions (to retrieve IDs), enabling more dynamic and powerful calculations and data displays. * **Output Package Support for Migrating Group Data:** Previously, migrating user groups between Veeva Vault environments required manual recreation. With 22R3, administrators can now include user groups in outbound packages, streamlining the migration process and reducing manual effort when moving configurations between sandboxes and production environments. * **Limit Workflow Participants:** This feature allows administrators to set a maximum number of participants for a specific role within a workflow start step. This prevents scenarios where a workflow task is assigned to an excessively large group (e.g., thousands of users), which could lead to operational inefficiencies. The default maximum is 5000, but it can be configured to a lower limit (e.g., 100). * **Workflow Participant Limit Error Handling:** If a workflow is initiated with a group that exceeds the configured maximum number of participants for a specific role, the system will now throw an error, preventing the workflow from starting. This ensures adherence to the defined participant limits and avoids unintended mass assignments. * **Document with Object Report Type:** This new report type enables users to create reports that use a document as the primary object and any related business object (e.g., Product, Country) as the secondary object. This allows for more comprehensive reporting that links document-centric data with other critical business information. * **Standard Report Type Object Limit:** When creating a standard report type in Veeva Vault, a maximum of 10 objects can be selected for inclusion. This is an important limitation to be aware of for report design and certification exam questions. * **Permissions for Glossary and Glossary Definition:** To view Glossary and Glossary Definition records in Veeva Vault, users require the "Content View Content" permission. This ensures controlled access to critical terminology and definitions within the system. Key Concepts: * **Veeva Vault 22R3 Release:** The latest major update to the Veeva Vault platform, introducing new features and enhancements. * **Veeva Vault Certification:** An examination validating an individual's knowledge and proficiency in configuring and managing Veeva Vault. * **Person Object:** A standard object in Veeva Vault representing individuals (e.g., users, external collaborators). * **Outbound Package:** A mechanism in Veeva Vault to package and migrate configurations, data, and components from one environment to another. * **Workflow:** An automated sequence of tasks and approvals within Veeva Vault, often used for document review, approval, or process management. * **Report Type:** A predefined structure in Veeva Vault that specifies which objects and fields are available for reporting, serving as the basis for creating reports. Examples/Case Studies: * **Duplicate Person Records:** The speaker provides an example of a user, Vaibhav Agrawal, having two accounts created due to lack of duplicate detection, leading to wasted licenses and administrative issues. The new feature resolves this by flagging potential duplicates during record creation. * **Email Notification Troubleshooting:** The speaker recounts personal experiences where he was unsure if a configured email notification was sent due to Outlook settings. The "Notification Email Notification Status" feature provides a direct way to verify delivery within Veeva Vault. * **Workflow Participant Overload:** An example is given where a workflow task might be assigned to a group of 1000 users, causing inefficiency. The "Limit Workflow Participants" feature allows setting a maximum (e.g., 100) to prevent such scenarios and ensure tasks are assigned to manageable groups.

Veeva Vault General Release 23R3 || Explained Document Features
Anitech Talk
/@AnitechTalk
This video provides an in-depth explanation of the document-related features introduced in the Veeva Vault 23R3 General Release. The presenter, speaking to Veeva Vault users, software enthusiasts, and those interested in tech developments, meticulously breaks down several key enhancements designed to improve document management, workflow efficiency, security, and user experience within the Veeva Vault platform. The core focus is on how these updates streamline operations, particularly in regulated environments like clinical and regulatory information management (RIM), by automating previously manual steps and providing more granular control over document processes. The presentation systematically covers a range of updates, starting with workflow automation for collaborative authoring sessions, which allows administrators to automatically initiate or conclude collaborative editing in Microsoft Office documents directly from workflow actions or entry actions. This addresses a common pain point of manual check-in/check-out processes. Following this, the video details improvements to the document viewer, such as a new right-click context menu for copying text, searching, and creating annotations, enhancing user interaction. Performance improvements are also highlighted, specifically a 70% increase in speed for rendering merge fields and bookmarks from Microsoft Word documents. Further into the release features, the video delves into critical enhancements for security and data integrity. Atomic security for Expected Document List (EDL) item actions is introduced, offering more refined control over specific actions within an EDL's lifecycle state, crucial for clinical and RIM users. This includes managing placeholders, generating documents from templates, uploading, matching, and for RIM, locking/unlocking or excluding/including documents. The intelligent document update feature is also discussed, ensuring that Vault only updates documents when actual changes are made, thereby minimizing unnecessary audit trail entries and promoting cleaner data. Other features covered include enhancements to updating the last match date on EDLs, flexible "send as link" version binding options (latest steady state, latest, or specific versions), improved document title display in binder compact view, and the ability for administrators to download previews of basic signature page templates without needing to apply an e-signature. Key Takeaways: * **Automated Collaborative Authoring Workflows:** The 23R3 release enables administrators to configure workflow action steps and entry actions to automatically initiate or conclude collaborative authoring sessions in Microsoft Office. This eliminates manual steps like check-out/check-in, significantly improving the smooth user experience and efficiency for teams working on shared documents. * **Enhanced Document Viewer Functionality:** Users now benefit from an improved document viewer context menu, accessible via right-click. This menu provides quick access to actions such as copying text, searching the glossary, and creating annotations directly within the document viewer, streamlining review and interaction. * **Significant Performance Boost for Document Rendering:** The update delivers a 70% faster rendering speed for merge fields and bookmarks within Microsoft Word documents. This improvement in processing tokens and bookmarks enhances productivity, especially for users frequently working with complex documents containing many dynamic fields. * **Granular Atomic Security for EDL Actions:** Veeva has introduced atomic security for Expected Document List (EDL) item actions, providing more precise control over specific operations within the EDL lifecycle. This is particularly beneficial for clinical and RIM users, allowing for fine-grained management of actions like creating placeholders, generating documents from templates, uploading, matching, and for RIM, removing, locking, or unlocking document versions. * **Optimized EDL Last Match Date Updates:** The system now intelligently updates the "last match date" field on EDLs only when a matching job actually results in matching or unmatching a document. This enhancement minimizes the number of audit entries logged, leading to cleaner audit trails and better data management. * **Flexible Document Link Sharing with Version Binding:** Users can now choose from three version binding options when sending a document link: "latest steady state version" (default for approved/effective documents), "latest version" (regardless of state), or "specific version." This flexibility ensures recipients access the appropriate document version, enhancing control and communication. * **Improved Document Visibility in Binder Compact View:** The binder compact view now displays the document title alongside the document name and number. This added visibility aligns the presentation with the library's detailed view, improving the overall document viewing experience and making it easier to identify documents within binders. * **Pre-downloadable Basic Signature Page Templates:** Administrators gain the capability to download previews of basic signature page templates. This feature streamlines the evaluation of template changes, allowing admins to review and confirm modifications without the need to apply a new e-signature to a test document, saving time and effort. * **Intelligent Document Update for Audit Trail Efficiency:** Veeva Vault 23R3 ensures that documents are only updated when actual changes are made, preventing the system from updating the "last modified date" and generating unnecessary audit trail entries when a document is saved without modification. This is crucial for data cleanup and reducing excess audited entries, especially for integrations or custom SDK code. * **Enhanced Regulatory Compliance and Auditability:** Features like atomic security for EDLs, optimized last match date updates, and intelligent document updates directly contribute to better regulatory compliance. They provide more robust control, reduce unnecessary audit trail clutter, and ensure that document management processes adhere to stringent industry standards like GxP and 21 CFR Part 11. **Tools/Resources Mentioned:** * Veeva Vault (specifically 23R3 General Release) * Microsoft Office Word (for collaborative authoring) * Official Veeva Vault 23R3 release notes (link provided in description) **Key Concepts:** * **Collaborative Authoring:** The ability for multiple users to work simultaneously on a single document, typically in Microsoft Office applications, integrated with Veeva Vault. * **Document Viewer Context Menu:** A right-click menu within the Veeva Vault document viewer that provides quick access to common actions like copying text, searching, and annotating. * **Merge Fields and Bookmarks:** Dynamic placeholders within Microsoft Word documents that can be populated with data from Veeva Vault, often used for generating standardized documents. * **Atomic Security:** A granular security control mechanism that allows for specific actions on document items (e.g., within an EDL) to be controlled based on user roles and lifecycle states. * **Expected Document List (EDL):** A feature in Veeva Vault (especially in Clinical Vault) used to track and manage anticipated documents for a study or project, ensuring all required documents are collected. * **RIM (Regulatory Information Management):** A suite of Veeva products focused on managing regulatory submissions, product registrations, and other regulatory content. * **Version Binding:** A feature that allows users to specify which version of a document (e.g., latest steady state, latest, or a specific version) a shared link will point to. * **Steady State Version:** Refers to a major, approved, or effective version of a document within Veeva Vault, typically representing a finalized and controlled state. * **Binder Compact View:** A display option within Veeva Vault binders that provides a more condensed view of documents, now enhanced to include document titles. * **Basic Signature Page Template:** A template used in Veeva Vault for generating electronic signature pages, which can now be previewed by administrators. * **Intelligent Document Update:** A system behavior where Veeva Vault only records a document modification and updates its "last modified date" when actual content changes occur, preventing unnecessary audit trail entries from simple saves without modification.

Veeva Vault API || Veeva APIs || All about Veeva APIs || How to use Veeva Vault APIs || Veeva Vault
Knowledge Srot
/@knowledgesrot
This video provides a practical, step-by-step guide on how to interact with Veeva Vault APIs, with a primary focus on the crucial initial step of generating a session ID for authentication. The presenter addresses common queries regarding Vault APIs, aiming to demystify their usage for developers and technical users. The tutorial begins by establishing the necessity of a session ID as the gateway to performing any operations within Veeva Vault via its APIs, such as querying or manipulating various Vault objects like binders, documents, users, SCIM groups, and picklists. The progression of the video meticulously walks viewers through the process of accessing the Veeva Vault Developer Forum, which serves as the official documentation hub for all Veeva Vault APIs. It highlights the availability of different API versions, specifically mentioning 23.1 as the latest and 21.1 (or 21.2) as the version used for the demonstration. A key aspect of the tutorial involves leveraging Postman, an API development environment, to streamline the API interaction. The presenter demonstrates how to import the entire Veeva Vault API collection directly into Postman from the developer forum, significantly simplifying the setup for users. The core of the demonstration revolves around the authentication process. The presenter explains that generating a session ID requires a POST request, specifying necessary headers like 'Content-Type' and 'Accept', and body parameters including a valid Veeva Vault username, password, and the Vault DNS (the specific URL of the Vault application). The video details how to input these credentials into Postman, emphasizing the importance of correctly identifying the Vault DNS and the API version. Upon successful authentication, the API returns a session ID along with other relevant details such as Vault IDs, the associated application names, and the user ID, which are then used for subsequent API calls to authorize requests for data retrieval or manipulation. The video concludes with a brief example of how to use the generated session ID to retrieve document fields, illustrating its application as an authorization token in the headers of subsequent GET requests. Key Takeaways: * **Session ID is Foundational:** The first and most critical step for any interaction with Veeva Vault APIs is to generate a session ID, which acts as an authentication token for all subsequent API calls. Without it, no other Vault API operations can be performed. * **Official Developer Resources:** The Veeva Vault Developer Forum (developer.veevavault.com) is the authoritative source for all Veeva Vault API documentation, including API versions (e.g., 23.1, 21.1) and detailed request/response structures. * **Postman for API Management:** Postman is an indispensable tool for interacting with Veeva Vault APIs. The video demonstrates how to import the entire Veeva Vault API collection directly into Postman, simplifying the process of making requests and managing API endpoints. * **Authentication Request Details:** Generating a session ID involves a POST request to the authentication endpoint, requiring specific headers (Content-Type, Accept) and body parameters: username, password, and the Vault DNS (the specific URL of the Veeva Vault instance). * **Understanding Vault DNS and Versioning:** Users must correctly identify their specific Vault DNS (e.g., for RIMS, Quality Vault, PromoMats) and the API version they intend to use (e.g., v21.1) to ensure successful API calls. * **Session ID as Authorization Token:** Once generated, the session ID is used in the 'Authorization' header of subsequent API requests to authenticate and authorize access to various Vault objects and functionalities. * **Querying Various Vault Objects:** Veeva Vault APIs allow programmatic interaction with a wide range of objects, including binders, documents, users, SCIM groups, and picklists, enabling comprehensive data management and integration. * **VQL for Data Retrieval:** The video mentions Veeva Query Language (VQL), which is analogous to SQL, for querying data within Veeva Vault, indicating robust data retrieval capabilities through the APIs. * **Successful Authentication Response:** A successful login returns a JSON response containing the session ID, associated Vault IDs, application names, and the user ID, all of which are crucial for further API interactions. * **Practical Application for Document Fields:** The video provides a concrete example of using the generated session ID to retrieve document fields, demonstrating a common use case for accessing metadata related to documents stored in Veeva Vault. Tools/Resources Mentioned: * **Veeva Vault Developer Forum:** (https://developer.veevavault.com) – The official portal for Veeva Vault API documentation and resources. * **Postman:** An API platform used for building, testing, and modifying APIs. The demonstration uses the web application version, but a desktop application is also available. * **Veeva Vault APIs:** Specific versions 21.1 and 23.1 are mentioned, indicating the importance of version control in API interactions. Key Concepts: * **Veeva Vault APIs:** Programmatic interfaces that allow external applications to interact with and manage data within Veeva Vault, a content and data management platform widely used in life sciences. * **Session ID:** A unique, temporary token issued upon successful user authentication, which must be included in subsequent API requests to verify the user's identity and authorization. * **Vault DNS:** The Domain Name System (DNS) address or URL that uniquely identifies a specific instance of a Veeva Vault application (e.g., a Quality Vault or RIMS Vault). * **VQL (Veeva Query Language):** A proprietary query language developed by Veeva, similar in syntax and function to SQL, used for retrieving specific data from Veeva Vault databases via APIs.

Sr. Clinical Research Associate Gets Candid On Clinical Research Career and Avoiding Traps
Dan Sfera
/@dansfera
This video provides an in-depth exploration of career development within clinical research, featuring a candid discussion between host Dan Sfera and guest Jasmyn Adams, a Senior Oncology Clinical Research Associate (CRA) and career coach. The conversation delves into Jasmyn's personal journey, highlighting the unconventional paths available in the industry, the importance of practical experience over excessive academic credentials, and the strategic necessity of personal branding and career management. A significant portion of the discussion also touches upon the operational realities of clinical trial sites and Contract Research Organizations (CROs), including the role of technology like Veeva Site Vault. The discussion begins with a notable shout-out to Veeva for sponsoring the podcast, specifically highlighting Veeva Site Vault's free offering for clinical sites to manage e-regulatory documents. This segues into Jasmyn's career trajectory, starting from her decision to leave a PhD program with a Master's degree to pursue clinical research, driven by a desire for a more direct impact on science, medicine, and patients. She recounts her early experiences, including a brief but impactful stint at an SMO (Site Management Organization) that engaged in questionable, even fraudulent, data practices, which she quickly left due to ethical concerns. This experience underscores the critical importance of regulatory compliance and data integrity within the industry. The conversation further progresses to Jasmyn's extensive experience across various large CROs, where she advanced from CTA (Clinical Trial Assistant) to CRA and eventually to CTM (Clinical Trial Manager), though she expresses a preference for the CRA role due to its direct impact and higher earning potential at certain levels. A key theme that emerges is the concept of "job-hopping" as a legitimate career growth tool in an industry where internal promotions can be slow. Jasmyn emphasizes that individuals are responsible for their own career development, not their employers. This leads to a discussion on the "institutionalized education shock," where many aspiring professionals believe more degrees and certifications are the answer, often overlooking the value of hands-on experience and soft skills. Jasmyn's coaching philosophy, "Confessions of a CRA," focuses on shifting from a "job mindset" to a "career mindset," teaching clients how to create a "job attraction system" through robust personal branding, marketing, and effective negotiation. Key Takeaways: * **Veeva Site Vault as an Enabler:** Veeva Site Vault is offered free to clinical sites, enabling them to digitize e-regulatory documents and passively share them with CRAs and sponsors. This streamlines operations, reduces manual tasks like emailing documents, and enhances data accessibility for Trial Master Files (TMFs). * **Unconventional Career Paths in Clinical Research:** The industry lacks a single, clear-cut path like medicine or law. Success often comes from diverse experiences and strategic career moves, rather than solely academic progression. * **Prioritizing Experience Over Excessive Degrees:** Many professionals are "over-educated" with multiple degrees but lack practical experience. Gaining hands-on experience at a clinical site, even through volunteering, is often more beneficial for career advancement than pursuing additional academic qualifications. * **Ethical Compliance is Paramount:** Early career experiences can expose individuals to unethical practices, such as data fabrication at SMOs. It is crucial for professionals to recognize and disengage from such environments to protect their personal livelihood and the integrity of clinical research. * **Job-Hopping as a Strategic Career Tool:** In the absence of rapid internal promotions, strategically moving between companies can be an effective way to gain diverse experience, accelerate career growth, and increase earning potential. * **Personal Responsibility for Career Development:** Individuals must take ownership of their career growth, as companies often prioritize their own objectives (e.g., shareholder profits) over individual employee development. * **The Value of Personal Branding and Marketing:** Developing a strong personal brand, marketing skills, and the ability to "sell yourself" are critical for career attraction. This includes optimizing LinkedIn profiles, crafting effective resumes and cover letters, and networking. * **Challenges of Large CROs vs. Mid-sized Companies:** Large CROs can offer less personalized onboarding, a feeling of being "just a number," and a focus on company-centric compliance training. Mid-sized companies often provide a more supportive culture, better career development resources, and closer access to decision-makers. * **Importance of Soft Skills and Mindset:** Interview anxiety, limiting beliefs, and a lack of confidence are significant barriers to career success. Coaching on mindset, communication, and negotiation skills is essential for professionals. * **Negotiation Gaps, Especially for Women:** Many professionals, particularly women, fail to negotiate for what they want (salary, promotions, benefits). Developing negotiation skills and overcoming the fear of asking are crucial for maximizing career potential. * **CRA Metrics and Performance:** Key performance indicators for CRAs at CROs typically include report turnaround times (first draft within 5 business days, final within 10-15 business days) and timely completion of mandatory training (both study-specific and corporate compliance). * **Bringing Value as an Intern/Volunteer:** When seeking entry-level positions or internships, it's vital to demonstrate value by doing homework, understanding basics, and actively contributing to day-to-day operations rather than just expecting to "shadow." **Tools/Resources Mentioned:** * **Veeva Site Vault:** A platform for e-regulatory document management for clinical sites, offered free by Veeva. * **LinkedIn:** Emphasized as a crucial tool for personal branding, networking, and job attraction. * **The Clinical Trials Guru (Dan Sfera's platform):** Host's channel/podcast, offering content on clinical trials. * **Confessions of a CRA (Jasmyn Adams' podcast/coaching):** Jasmyn's platform for CRA career coaching and development. **Key Concepts:** * **Institutionalized Education Shock:** A term used to describe the phenomenon where individuals are so ingrained in the belief that more education (degrees, certifications) is always better, that they overlook practical experience and delay career progression. * **Job Attraction System:** A strategy for career development that focuses on personal branding, marketing, and networking to attract job opportunities rather than solely relying on traditional job applications. * **BMS (Branding, Marketing, Selling):** Jasmyn's acronym for the essential skills needed to advance one's career in the competitive clinical research landscape. * **Equity (in a capitalistic society):** Defined as ownership in a business or idea, contrasting with the common misconception of equity solely as fairness or equality in compensation. **Examples/Case Studies:** * **Fraudulent Data at an SMO:** Jasmyn recounted an early experience at an SMO where data was fabricated (e.g., non-existent patients, tweaked lab values) to meet enrollment criteria and secure budget payments, leading her to resign due to ethical concerns. * **Onboarding at Large vs. Mid-sized CROs:** Jasmyn contrasted the impersonal, self-directed onboarding process at large CROs (receiving equipment with minimal guidance) with the more supportive, structured, and personalized onboarding at mid-sized companies, which included daily check-ins and comprehensive development resources. * **Employer Conflict over Personal Branding:** Jasmyn shared an experience where a previous employer gave her an ultimatum to shut down her coaching business and podcast or resign, highlighting the potential conflict between corporate interests and individual branding, and reinforcing the need for personal career autonomy.

Veeva Summit Keynote: Veeva Development Cloud Vision
Veeva Systems Inc
/@VeevaSystems
This video keynote from Veeva Summit outlines Veeva's strategic vision for building an integrated "industry cloud" for life sciences, the Veeva Development Cloud, which spans the full product lifecycle from clinical to regulatory, quality, and safety on the Vault platform. The speaker details significant platform enhancements, updates across their application suites, and introduces the ambitious Digital Trials Platform. The discussion also touches on Veeva's recent conversion to a Public Benefit Corporation, emphasizing a long-term commitment to balancing stakeholder interests. A key segment features an interview with Jörg Möller of LEO Pharma, highlighting their partnership with Veeva on digital trials and the anticipated benefits of shortening development timelines and reducing costs. Key Takeaways: * Veeva's overarching strategy is to create a unified industry cloud for life sciences, integrating clinical, regulatory, quality, and safety operations on the Vault platform to enhance efficiency and effectiveness across the product lifecycle. * Recent Vault platform enhancements include Microsoft Office integration with collaborative authoring and Excel report templates, the upcoming Vault Mobile app for secure document access, and the new Action UI for a more productive and modern user experience. * The Vault Quality suite is expanding with new offerings like Vault Validation Management for digital validation processes, Vault LIMS for optimizing quality control, and Veeva Learn GxP, a content library of accredited e-learning courses for GxP training. * Veeva's Clinical (CTMS, EDC, CDB) and Safety solutions are maturing rapidly, demonstrating strong adoption and enterprise-wide implementation by major pharmaceutical companies, reflecting the industry's need for integrated solutions in these complex areas. * A major multi-year initiative is the Veeva Digital Trials Platform, designed to fundamentally improve clinical trial information flow by connecting sponsors, patients, and research sites through applications like Site Connect, e-Consent, e-PRO, virtual visits, e-Source, and sensor data, aiming to enhance patient experience and streamline operations. * Partnerships with early adopter customers, such as LEO Pharma's collaboration on the digital trials platform, are crucial for product development and validation, with LEO Pharma targeting a 25% reduction in clinical development timelines and costs. * Veeva's conversion to a Public Benefit Corporation reinforces its commitment to a balanced, long-term approach that prioritizes customer and employee success alongside shareholder value, particularly as technology becomes increasingly vital to life sciences.

2023 Veeva R&D and Quality Summit Opening Keynote Ft. Replimune and Gilead
Veeva Systems Inc
/@VeevaSystems
This video provides an in-depth exploration of Veeva's vision, technological innovations, and product roadmap for its R&D and Quality Summit in 2023. Peter Gassner, Veeva CEO, sets the stage by outlining Veeva's commitment to building the "industry Cloud for Life Sciences," encompassing software, data, and services designed to enhance efficiency and effectiveness across the sector. He emphasizes Veeva's unique status as a Public Benefit Corporation (PBC), prioritizing the balanced interests of customers, employees, shareholders, and the industry over mere shareholder value, positioning Veeva as a durable, long-term partner. A significant portion of the keynote addresses technology trends, particularly the rise of Artificial Intelligence (AI) and Large Language Models (LLMs). Gassner offers a pragmatic view, asserting that while AI applications will become powerful tools, they will not replace humans or create lasting competitive advantage on their own. Instead, Veeva's approach to AI is focused and practical, centered on two key areas: "application Bots" designed to automate repetitive, high-volume functions within specific applications (e.g., TMF bot for document classification, RIMbot) and a "Direct Data API." This groundbreaking API, developed over two years, promises to extract full and incremental data from Veeva Vault applications up to 100 times faster than existing methods, enabling customers and partners to more easily build their own AI applications requiring complete and current data views. The presentation then delves into the Veeva Development Cloud, which serves as the technological foundation for drug development across clinical, regulatory, safety, and quality domains. Gassner highlights the comprehensive nature of Veeva's Clinical Platform, aiming to be the most complete and highest quality solution for patients, sites, and sponsors, with over 30 applications including seven new ones announced. Key innovations include SiteVault for site collaboration, Study Training, randomization and trial supply management (RTSM), e-PRO for patient-reported outcomes, and new industry data areas like SiteBase and Open Data Clinical. A major focus is on improving the site experience through free software like SiteVault, and the introduction of Veeva ID and Study Portal to simplify login and system access for clinical research coordinators who often manage dozens of different credentials. The Regulatory, Safety, and Quality platforms are also detailed, with emphasis on continuous improvement, increased automation, and the development of unified systems like the Quality Platform for manufacturing and R&D, including new applications such as e-forms and Batch Release. The keynote concludes with insights into Vault Platform innovations, such as "action layouts" for streamlined user interfaces and a significant reduction in upgrade downtime (aiming for 10 minutes or less). Customer interviews with Replimune (an emerging biotech) and Gilead (an enterprise pharma) illustrate how companies are leveraging Veeva's platform approach to unify processes, reduce manual work, and drive efficiency, with Replimune citing a 30% reduction in site data entry burden and significant cost savings. Finally, Veeva introduces "Veeva Business Consulting," a new service offering deep industry expertise to help clients align software investments with strategic business objectives, focusing on process optimization, change management, and value realization. Key Takeaways: * Veeva's foundational vision centers on building an "industry Cloud for Life Sciences" that integrates software, data, and services, aiming to be essential and appreciated by every company in the sector. Their Public Benefit Corporation (PBC) status underscores a commitment to balancing the interests of all stakeholders for long-term industry benefit. * Veeva adopts a "focused and practical approach" to AI, viewing it as a powerful tool rather than a human replacement or a standalone competitive advantage. Their strategy involves embedding AI into specific applications to automate repetitive tasks. * "Application Bots" are a core component of Veeva's AI strategy, designed to perform specific, high-volume functions within applications, such as the TMF bot for document classification and the new RIMbot, freeing human users for more complex work. * The "Direct Data API" is a groundbreaking innovation, enabling extremely fast extraction of full and incremental data from Veeva Vault applications (up to 100 times faster). This is crucial for customers and partners to build their own AI applications and robust data pipelines that require complete and current data. * Veeva's Clinical Platform is designed to be comprehensive and high-quality, serving the entire clinical ecosystem by being patient-centric, site-centric, and sponsor-centric. It includes a wide array of applications from eTMF and CTMS to RTSM, ePRO, and new industry data solutions. * Improving the site experience is a key focus, with initiatives like free SiteVault software, Veeva ID (a single login for all Veeva systems across sponsors), and the Study Portal, which collectively aim to alleviate the burden of managing multiple logins and systems for clinical research sites. * Veeva's Regulatory and Quality platforms are mature and continuously evolving, with features like collaborative authoring in Microsoft Word, continuous publishing, and unified quality systems across QA, QC, and training, demonstrating ongoing innovation even in established areas. * The Safety and Quality areas are seeing significant investment, with new applications like Safety Workbench, Signal (for analytics), e-forms (to replace paper-based processes), and Batch Release (for faster product release decisions), all aimed at increasing automation, efficiency, and data-driven insights. * Vault Platform innovations include "action layouts," which provide highly optimized, role-specific views to streamline user workflows and training, and a commitment to "10-minute upgrades" to minimize downtime and ensure world-class availability for critical operations. * Customer case studies highlight the tangible benefits of Veeva's platform approach: Replimune, an emerging biotech, achieved a 30% reduction in site data entry burden and nearly $1 million in monitoring cost savings per trial by pooling vendor and EDC data in Vault CDB. * Gilead, an enterprise pharma, demonstrated an evolutionary approach, leveraging Veeva QualityDocs and QMS to unify processes, reduce risk, improve visibility, and align business processes globally, emphasizing the value of coupling technology modernization with process improvements. * Veeva Business Consulting is a new strategic service offering, providing deep industry expertise to help clients optimize business processes, navigate change journeys, and ensure value realization from their software investments, bridging the gap between technology and strategic objectives. * Veeva positions itself as a conduit for industry collaboration, fostering a community where companies can share ideas and collectively drive positive change in life sciences through better ways of working. Tools/Resources Mentioned: * Veeva Vault (overall platform) * Veeva Development Cloud (suite for R&D and Quality) * Veeva Commercial Cloud (mentioned as a separate offering) * Application Bots (TMF bot, RIMbot) * Direct Data API * Veeva Clinical Platform (eTMF, CTMS, SiteVault, Study Training, RTSM, ePRO, SiteBase, TrialBase, Open Data Clinical) * Veeva ID * Study Portal * Veeva Regulatory Platform (publishing, archive viewer) * Veeva Safety Platform (Safety, SafetyDocs, Safety Workbench, Signal) * Veeva Quality Platform (QualityDocs, QMS, Training, Validation Management, LIMS, e-forms, Batch Release) * Vault CDB (Clinical Data Management) * Microsoft Word (for collaborative authoring) * SAP (for integration with Batch Release) Key Concepts: * **Industry Cloud for Life Sciences:** Veeva's vision of providing comprehensive software, data, and services tailored specifically for the life sciences sector. * **Public Benefit Corporation (PBC):** A legal status indicating a company's commitment to balancing stakeholder interests (customers, employees, shareholders, industry) beyond just maximizing shareholder value. * **Application Bots:** AI-powered tools embedded within specific software applications to automate repetitive, high-volume tasks. * **Direct Data API:** A high-speed, foundational API designed to extract complete and incremental data from Veeva Vault applications, enabling external AI development and data integration. * **Clinical Ecosystem:** The interconnected network of patients, research sites, and sponsors involved in clinical trials, emphasizing the need for platforms that serve all three. * **Site-centricity:** A focus on designing solutions that cater to the needs and challenges of clinical research sites, improving their experience and efficiency. * **Unified Quality System:** An integrated approach to managing quality across quality assurance, quality control, and training within manufacturing and R&D. * **Action Layouts:** Specialized, optimized user interface views within Veeva Vault applications, tailored to specific activities and user roles to streamline workflows. Examples/Case Studies: * **Replimune:** An emerging biotech that utilized Vault CDB to pool vendor and EDC data, resulting in a 30% reduction in site data entry burden and an estimated savings of almost $1 million per trial across three phase two oncology trials. * **Gilead:** An enterprise pharmaceutical company that implemented Veeva QualityDocs and QMS in an evolutionary manner, starting with external partner collaboration and expanding to internal document management. They focused on aligning business processes globally, leveraging Veeva's industry best practices to achieve risk reduction and improved visibility.