Videos
Browse videos by topic
All Videos
Showing 313-336 of 345 videos

Veeva Systems CEO: Chasing the Cloud | Mad Money | CNBC
CNBC
/@CNBC
Jun 2, 2017
This video provides an in-depth exploration of Veeva Systems' strategic position and growth within the cloud computing landscape, specifically tailored for the pharmaceutical, biotech, and life sciences industries. Jim Cramer interviews Peter Gastner, co-founder and COO of Veeva Systems, to discuss the company's recent financial performance, its unique market approach, and future prospects. The conversation highlights how Veeva has successfully carved out a niche by bringing the benefits of cloud computing to highly specialized industry applications, a strategy that Gastner asserts is both innovative and highly effective. The discussion delves into Veeva's comprehensive suite of cloud-based software solutions, which address critical operational needs across the life sciences value chain. These applications range from enhancing the effectiveness of pharmaceutical sales representatives and streamlining data capture for clinical trials to ensuring robust compliance with government regulations. Gastner emphasizes that despite the competitive nature of the cloud market, Veeva's differentiated strategy of deep industry specificity allows it to be a strategic partner for its customers, including major players like Pfizer and Novartis, as well as smaller, emerging biotechs. A significant portion of the interview addresses the macro trend of cloud computing, with Gastner agreeing with other tech leaders that the industry is still in its "early days" and will continue to expand over the next two to three decades. He counters skepticism about Veeva's total addressable market (TAM), stating that the life sciences market alone represents a $7 billion opportunity, which Veeva continuously expands by introducing new products. Gastner also cites Gartner's analysis, which identifies industry-specific applications as the largest and fastest-growing segment of cloud computing, a $132 billion market that is twice the size of ERP and CRM combined. This reinforces Veeva's long-term growth potential and its resilience against broader economic or regulatory shifts. The video also touches upon Veeva's financial strength, noting its consistent delivery of "30/30 quarters," characterized by over 30% revenue growth and 30% profit. This robust performance is attributed to the company's extensive product portfolio and its ability to serve as a strategic, long-term partner to its diverse customer base. The interview concludes with a strong endorsement of Veeva's momentum and earnings growth, positioning it as a leading example of a successful cloud company in a specialized market. Key Takeaways: * Veeva Systems has successfully implemented a differentiated strategy by focusing exclusively on providing cloud-based software for the pharmaceutical, biotech, and life sciences industries, distinguishing itself from broader cloud competitors. * The company's product offerings are comprehensive, designed to optimize various critical functions within life sciences, including improving pharmaceutical sales force effectiveness, accelerating clinical trials through efficient data capture, and ensuring adherence to government regulations. * Veeva's "Vault regulatory information management" is highlighted as a key solution, assisting major pharmaceutical companies like Merck in automating regulatory processes required for product registration and compliance. * Cloud computing is presented as a long-term, macro-level trend still in its nascent stages, with significant growth expected over the next 20-30 years, underscoring the enduring market opportunity for specialized cloud solutions. * Veeva serves as a strategic partner to a wide range of clients, from large pharmaceutical enterprises such as Pfizer and Novartis to smaller, emerging biotechs, by offering a complete and integrated suite of products. * New biotechs commercializing their first products often opt for a full suite of Veeva products from inception, bypassing legacy client-server systems to leverage integrated solutions and achieve faster time-to-market. * Concerns regarding potential deregulation impacting Veeva's regulatory compliance software are dismissed, as the company operates globally and supports customer innovation and effectiveness irrespective of specific policy changes. * The total addressable market (TAM) for Veeva within the life sciences sector is substantial, estimated at $7 billion, and the company actively expands this market by continuously introducing new applications, with 8 of its 24 products launched in the past year. * According to Gartner, industry-specific applications constitute the largest and fastest-growing segment of cloud computing, representing a $132 billion market that grew 10% last year, making it twice the size of the combined ERP and CRM markets. * Veeva demonstrates strong financial performance, consistently achieving "30/30 quarters" (over 30% growth in both revenue and profit), a testament to its extensive product portfolio and strategic customer relationships. * The video underscores the market's appreciation for companies with strong momentum and consistent earnings growth, positioning Veeva as a prime example of a successful, specialized cloud provider. * Veeva's success validates the power of a targeted, industry-specific strategy combined with continuous innovation, proving that a focused approach can lead to significant market leadership and expansion within a niche. Tools/Resources Mentioned: * Veeva CRM * Veeva Vault regulatory information management * Gartner (for market analysis) Key Concepts: * **Cloud Computing:** The delivery of on-demand computing services—including servers, storage, databases, networking, software, analytics, and intelligence—over the Internet ("the cloud"). * **Industry-Specific Applications:** Software solutions designed and tailored to meet the unique needs and regulatory requirements of a particular industry, such as pharmaceutical and life sciences. * **TAM Expanders:** Companies that not only capture existing market share but also expand the total addressable market by introducing new products or services that create new demand. * **30/30 Quarter:** A term used to describe a company's financial quarter where both revenue growth and profit growth exceed 30%, indicating strong performance and profitability. Examples/Case Studies: * **Merck:** Cited as a client utilizing Veeva for marketing communications, field force automation, clinical trials, and regulatory assistance. * **Pfizer and Novartis:** Mentioned as examples of large pharmaceutical companies that are Veeva customers. * **Small Biotechs:** Highlighted as a growing client segment that benefits from Veeva's integrated suite for speed to market.

Xybion Software Demo - Quality Management System For Opentext
XybionVideos
/@XybionVideos
Apr 5, 2017
This video provides an in-depth demonstration of Xybion's Quality Management System (QMS) built on the OpenText platform, showcasing its capabilities for companies operating in highly regulated industries, particularly the life sciences sector. Presented by Sunil Tiwari, Director of Technical Operations and Compliance Solutions at Xybion, the session walks viewers through the core functionalities of the QMS product and how it facilitates comprehensive quality management directly within the OpenText environment. The primary objective is to illustrate how this integrated solution drives operational efficiency, ensures regulatory compliance, and streamlines critical quality processes. The QMS solution is designed to manage essential quality processes such as deviations, Corrective and Preventive Actions (CAPAs), non-conformances, and change control. It leverages OpenText's inherent capabilities for imaging, document management, and workflow, providing a graphical and intuitive interface for end-users to configure quality management workflows. A key highlight is its compliance with major regulatory standards, including 21 CFR Part 11, a critical requirement for the life sciences sector. The system boasts high configurability, allowing organizations to tailor workflows to their specific needs rather than adopting a rigid, one-size-fits-all approach, which is crucial given the subtle differences in business processes across organizations. The demonstration focuses on a sample CAPA workflow, starting from the logging of a safety incident. The process unfolds through several stages: an initial observation, creation of a CAPA, investigation by a designated individual, and ultimately, the closure of the CAPA. While the full workflow including root cause analysis and detailed action plans is configurable, the demo streamlines it to show the core navigation and decision points. Throughout the process, the system facilitates role-based assignments, automated email notifications, and maintains a clear hierarchy of linked items (e.g., CAPA linked to observation). The presenter emphasizes features like automated ID generation for documents and records, configurable dashboards for real-time monitoring of CAPA statuses, and the ability to attach evidence documents with templated naming conventions. A significant portion of the demonstration highlights the system's robust e-signature capabilities, which are essential for regulatory compliance. The e-signature process involves multiple signatories (e.g., CAPA owner, safety reviewer, CAPA closer) and generates a PDF rendition that captures the signatures, date, time stamp, meaning of the signature, and title, along with all relevant form information from the CAPA process. This ensures an undeniable audit trail. Furthermore, the QMS includes comprehensive audit trails for all configuration changes, recording old and new values, timestamps, and user information. The system also supports multi-browser and multi-language functionality, offers various reporting formats (HTML, PDF, Word, Excel, CSV), and provides tools for exporting and importing configurations between different environments (e.g., QA to production), simplifying deployment and maintenance. Key Takeaways: * **Integrated QMS for Regulated Industries:** The Xybion QMS is specifically designed for companies in highly regulated sectors, particularly life sciences, to manage critical quality processes like CAPAs, deviations, non-conformances, and change control. * **Leveraging OpenText Platform:** The solution directly integrates with the OpenText Content Server, utilizing its document management, security, workflow, and forms capabilities, which can lead to reduced support costs, lower user training, and faster deployment. * **21 CFR Part 11 Compliance:** A core feature of the QMS is its adherence to major regulatory standards, explicitly including 21 CFR Part 11, which is vital for electronic records and signatures in the pharmaceutical and life sciences industries. * **Highly Configurable Workflows:** The system is 100% configurable, allowing organizations to customize business process workflows (e.g., CAPA, root cause analysis) to match their unique operational complexities and requirements, avoiding a rigid, generic approach. * **Automated ID Generation and Document Control:** The QMS includes an automated ID generator for unique identification of observations, CAPAs, and other records, along with the ability to apply ID templates to uploaded documents, streamlining naming conventions and document management. * **Comprehensive Audit Trails:** The system maintains detailed audit trails for all configuration changes and workflow actions, capturing old and new values, date/time stamps, and user information, which is crucial for regulatory scrutiny and accountability. * **Robust E-Signature Capabilities:** Electronic signatures are fully integrated for legally binding approvals and sign-offs. These signatures are captured with associated metadata (date, time, meaning, title) and rendered into a PDF, ensuring regulatory compliance for record integrity. * **Role-Based Assignments and Notifications:** Workflows support role-based assignments for tasks and trigger automated email notifications, ensuring that relevant stakeholders are informed and can act promptly on pending items. * **Actionable Dashboards and Reporting:** Configurable dashboards provide real-time visibility into the status of various quality processes (e.g., CAPA by observation type), allowing users to monitor progress and drill down into specific records. Reports can be generated in multiple formats (HTML, PDF, Word, Excel, CSV). * **Simplified Configuration Management:** The export and import functionality for configurations allows organizations to easily move system setups from development or QA environments to production, significantly reducing recreation effort and ensuring consistency. * **Multi-Language and Multi-Browser Support:** The application supports multiple languages and is tested on various browsers (e.g., IE11, Chrome), enhancing its usability and accessibility for global operations. * **Data Exchange Capabilities:** The QMS can exchange information with other tables or objects within the OpenText LiveLink environment, enabling lookups of organizational data or people, and fostering a more integrated enterprise data landscape. **Tools/Resources Mentioned:** * Xybion QMS (Quality Management System) * OpenText Content Server (specifically CS10 and CS10.5 versions) **Key Concepts:** * **Quality Management System (QMS):** A formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. * **CAPA (Corrective and Preventive Action):** A process for investigating and correcting non-conformances (corrective action) and preventing their recurrence (preventive action). * **21 CFR Part 11:** Regulations issued by the FDA that set forth requirements for electronic records and electronic signatures to be considered trustworthy, reliable, and equivalent to paper records and handwritten signatures. * **GRC Suite (Governance, Risk, and Compliance):** A comprehensive approach to managing an organization's overall governance, enterprise risk management, and compliance with regulations. * **Deviations:** Departures from approved instructions or established standards. * **Non-conformance:** A failure to meet a requirement. * **Change Control:** A formal process used to ensure that changes to a product, system, or process are introduced in a controlled and coordinated manner. * **E-signature:** Electronic data that is logically associated with other electronic data and which is used by the signatory to sign the electronic data. * **Audit Trail:** A chronological record of system activities, including who performed what action, when, and what the outcome was, crucial for regulatory compliance. **Examples/Case Studies:** * **Sample CAPA Workflow:** The video demonstrates a simplified CAPA workflow initiated by a "safety incident." The steps include logging the incident, creating an observation, generating a CAPA, assigning an investigator, the investigator's report, the CAPA owner's review, and finally, the closure of the CAPA with e-signatures from multiple parties. This example illustrates the system's ability to manage the lifecycle of a quality event from initiation to closure.

The latest from the FDA Preparing for the New Module 1 and Validation Criteria Recording 05122011
USDM Life Sciences
/@usdatamanagement
Jul 2, 2015
This video provides an in-depth exploration of the FDA's proposed changes to eCTD Module 1 and updated validation criteria, as presented in 2011. Harve Martin of expedo, a seasoned expert in life sciences information systems and a key figure in ICH M2 and IRISS, offers a unique perspective as a software designer tasked with implementing these complex regulatory requirements. The presentation aims to clarify the reasons behind varying eCTD validator outcomes and highlight critical areas for pharmaceutical companies to focus on as the FDA moved towards implementation of these new standards. The discussion begins by detailing the FDA's draft validation criteria version 2.0, released in December 2010, which introduced 58 new rules, removed 14, and significantly revised many existing ones. Martin explains the technical underpinnings of eCTD, emphasizing its XML basis and the role of DTDs (currently version 3.2) in defining rules. He highlights the challenges faced by software developers in interpreting and implementing ambiguous or technically impossible rules, citing specific examples of problematic criteria that could lead to submission rejections. A significant shift noted was the FDA's intention to become tougher on enforcement, with a heavy emphasis on document quality, the content validation of fillable forms, and robust PDF validation, including checks for broken or corrupt hyperlinks and bookmarks. Following the validation criteria, the presentation shifts to the anticipated changes in eCTD Module 1, which was not yet published but expected in draft form by July 2011. These changes aimed to address inconsistencies and improve granularity, particularly for CBER/CDER (DD Mac and CBER APLB) submissions. Key updates included reorganizing administrative information, allowing multiple applications per submission instance, and introducing more detailed headings and attributes for promotional materials to distinguish between professional and consumer audiences. Martin also outlines the FDA's internal "to-do list" for updating related guidance documents and specifications. He concludes with practical recommendations for companies, focusing on transition planning, impact on document lifecycle, reviewing SOPs, ensuring PDF compliance, and engaging with vendors and industry groups like IRISS to navigate these evolving regulatory landscapes. Key Takeaways: * **Evolving FDA Validation Criteria:** The FDA's draft validation criteria version 2.0 (circa 2010) introduced a substantial number of new rules (58 total, including 4 high-severity), removed problematic ones, and aimed for stricter, more uniform enforcement, particularly for high-severity issues that could lead to submission rejection. * **Technical Challenges in Implementation:** Software designers faced significant challenges in implementing validation rules due to ambiguities, technical impossibilities (e.g., "more than one version of the US Regional XML file exists"), or lack of clear definitions, leading to inconsistencies across different eCTD validators. * **Emphasis on Document and PDF Quality:** The new criteria placed a heavy emphasis on the quality of submitted documents, including the content validation of fillable forms downloaded from the FDA website and comprehensive PDF validation, checking for issues like broken hyperlinks, corrupt bookmarks, and proper embedded fonts. * **Module 1 Granularity and Multiple Applications:** The anticipated Module 1 changes aimed for greater granularity, especially in administrative information and promotional materials, and a significant departure from previous standards by allowing submissions to target multiple applications within a single submission instance. * **Impact on Lifecycle Management:** The increased granularity and structural changes in Module 1 were expected to have a significant impact on the lifecycle management of regulatory documents, requiring companies to re-evaluate their document authoring SOPs and potentially their content management systems. * **Importance of Industry Standards and Interoperability:** The presentation underscored the role of ICH M2 and the newly formed IRISS (Implementation of Regulatory Information Submission Standards) in fostering interoperability and addressing implementation challenges for electronic regulatory submissions across the industry. * **Strategic Planning for Transition:** Companies were advised to plan carefully for the transition to the new Module 1, considering its impact on existing processes, engaging with software vendors, conducting pilot submissions, and ensuring robust internal validation processes. * **PDF Compliance Beyond Basic Generation:** Beyond simply generating PDFs, companies needed to focus on the quality of hyperlinking, bookmarking, and embedded fonts, as these elements would be subject to more rigorous validation checks by the FDA. * **The Role of XML in eCTD:** The eCTD structure is fundamentally based on XML, with rules defined by DTDs (e.g., version 3.2). Understanding the XML backbone and its validation against DTDs is crucial for ensuring submission compliance. * **Collaboration and Continuous Feedback:** The iterative nature of regulatory updates (e.g., draft criteria, comment periods) highlighted the importance of industry feedback to the FDA and collaboration with vendors and peer organizations (like IRISS) to refine and improve submission standards. **Key Concepts:** * **eCTD (electronic Common Technical Document):** A standard format for submitting applications, amendments, supplements, and reports to regulatory authorities. * **Module 1:** The region-specific administrative information and prescribing information within an eCTD submission. * **Validation Criteria:** A set of rules and checks applied by regulatory authorities (like the FDA) to ensure the technical and structural compliance of eCTD submissions. * **XML (Extensible Markup Language):** The foundational language used for structuring eCTD submissions. * **DTD (Document Type Definition):** A set of markup declarations that define the legal building blocks of an XML document, used to validate the structure of eCTD files. * **ICH M2:** An expert working group within the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, focused on electronic standards. * **IRISS (Implementation of Regulatory Information Submission Standards):** A multi-industry, multinational, non-profit organization established to advance technical and electronic regulatory submission standards, with a focus on implementation and interoperability. * **RPS (Regulatory Product Submission):** A standard for electronic submissions that aims to provide a harmonized approach across different regulatory agencies and product types. * **DD Mac (Document Data Management Center) / CBER APLB (Center for Biologics Evaluation and Research, Advertising and Promotional Labeling Branch):** FDA centers/branches that accept eCTD submissions, specifically mentioned in the context of Module 1 changes.

eTMF
ePharmaSolutions
/@ePharmaSolutions
Aug 13, 2014
This video by ePharmaSolutions details their cloud-based Electronic Trial Master File (eTMF) solution, which is designed to streamline and accelerate the clinical development process for sponsors, Contract Research Organizations (CROs), and study sites. The solution focuses on efficient clinical document management, system integration with leading e-clinical platforms like CTMS and EDC, and ensuring regulatory compliance. Key features include configurable workflows, automated document routing, digital signatures, role-based access, and extensive reporting capabilities, all while reducing manual tasks and ensuring data integrity across thousands of studies and millions of documents. The system is built to be highly scalable, configurable, and extensible to external users, maintaining global security and compliance standards. Key Takeaways: * **Critical Role of Compliant Clinical Document Management:** The video highlights the necessity of a robust eTMF solution for managing vast quantities of clinical trial documents securely and compliantly, adhering to standards like the DIA 2.0 reference model. * **Automation and Efficiency in Clinical Operations:** The eTMF significantly reduces manual efforts through intelligent routing, auto-configuration, and automated workflows for document completion, approval, and QC. * **Interoperability and Data Integration:** The solution's robust integration APIs with industry-leading e-clinical vendors (CTMS, EDC, IVRS, lab systems) and ETL capabilities underscore the importance of a connected data ecosystem. * **Data-Driven Insights and Quality Assurance:** The eTMF offers comprehensive reporting (20+ out-of-the-box reports, ad hoc capabilities, milestone tracking) and advanced QC modules with configurable thresholds and bulk review.ai to apply its AI and LLM expertise. Potential applications include intelligent document classification, automated content extraction for compliance checks, AI-powered summarization of trial progress, and predictive analytics for document completion or quality issues.

EQMS software interview with Sparta Systems' Mohan Ponnudurai (QDL, 2-21-14)
Quality Digest
/@QualityDigest
Feb 24, 2014
This video provides an in-depth exploration of Enterprise Quality Management Software (EQMS), featuring an interview with Mohan Ponnudurai, Industry Solution Director at Sparta Systems. The discussion centers on the critical need for integrated quality processes, particularly in the context of complex supply chains where fragmented systems often lead to missing data, increased risks, and compromised product safety. Ponnudurai defines EQMS as a foundational system designed to harmonize disparate strategic systems like ERP, PLM, and LIMS, thereby centralizing and managing all quality processes globally. The core problem addressed is the prevalence of multiple, disconnected systems within companies, where key quality processes are performed in silos—ranging from pillar systems to manual spreadsheets. This fragmentation prevents the sharing of essential quality data, making it difficult to gain a holistic view of quality. EQMS aims to overcome this by integrating these systems, allowing for seamless data exchange and providing a unified platform to manage quality across three crucial dimensions: all quality processes, various functional business units (e.g., audit, QA, procurement), and unique geographic operating locations. This integration offers organizations unprecedented transparency and visibility into quality issues. Ponnudurai illustrates the practical application of EQMS with two relatable examples. First, in a manufacturing scenario, a deviation (like a part not fitting) triggers a record in the EQMS. The system automatically retrieves relevant information—such as serial number, lot number, date of manufacture, and supplier—from integrated manufacturing, ERP, or product master data systems. This ensures all necessary data is tied together rapidly for remediation. Second, in a customer-related example, a customer service representative logs a complaint (e.g., a missing part). The EQMS integrates with CRM to capture customer details and with ERP to pull product information, such as serial and lot numbers. This automated data input not only ensures accuracy and saves time but also enables quicker triage by immediately identifying if the problem is associated with a known lot number. Technically, EQMS is presented not as a replacement for existing critical systems but as a complementary solution. It connects to and leverages information from established "pillar systems" like ERP, PLM, LIMS, and document management, handling the end-to-end process of quality management in a centralized manner. This cohabitation allows companies to maximize their existing technology investments while gaining enhanced quality oversight. Furthermore, EQMS significantly improves reporting capabilities, enabling the generation of unique and comprehensive reports that were previously impossible due to data silos. By pulling relevant data from various sources, EQMS provides mid-level managers and top management with actionable insights into trends, performance benchmarks, and the effectiveness of fixes, facilitating timely and impactful decision-making. Key Takeaways: * **Fragmented Systems Hinder Quality:** Traditional supply chain monitoring often relies on disconnected software and manual processes, leading to data gaps, increased risks, and compromised product safety. * **EQMS Centralizes Global Quality:** Enterprise Quality Management Software (EQMS) serves as a pillar system that harmonizes fragmented strategic systems (e.g., ERP, PLM, LIMS) to manage all quality processes globally. * **Three Dimensions of Quality:** EQMS integrates quality management across processes, functional business units (e.g., audit, QA, procurement), and diverse geographic locations, providing a comprehensive view. * **Enhanced Transparency and Visibility:** By unifying quality data, EQMS offers organizations greater transparency and visibility into issues, enabling quicker reaction times and more effective problem-solving. * **Rapid Issue Resolution and Analysis:** EQMS helps companies solve issues quickly and analyze recurring problems or trends, allowing for the application of effective methodologies across different areas. * **Complementary Integration, Not Replacement:** EQMS is designed to complement and connect with existing critical systems (ERP, CRM, PLM, LIMS, document management) rather than replacing them, leveraging existing data and infrastructure. * **Automated Data Capture for Accuracy:** Integration with source systems ensures that critical information (e.g., serial numbers, lot numbers, supplier data) is automatically retrieved and accurately associated with quality events like deviations or complaints. * **Improved Triage and Decision-Making:** Real-time access to integrated data allows for quicker triage of issues, such as identifying if a customer complaint relates to a known problematic lot number, leading to faster remediation. * **Actionable Reporting and Analytics:** EQMS enables the generation of unique and comprehensive reports by consolidating data from various sources, providing mid-level and top management with actionable insights for impactful decisions. * **Data Utility Requires Information Delivery:** The video emphasizes that data, no matter how abundant, is useless unless it can be processed and presented as useful information to the right people at the right time. * **Supply Chain Quality is Paramount:** Effective monitoring of the supply chain's quality processes is crucial for reducing risks to the company and ensuring overall product safety. * **Time is Money in Quality Management:** The ability to rapidly find, react to, and remediate quality issues directly translates to cost savings and improved operational efficiency. Tools/Resources Mentioned: * Sparta Systems (company) * TrackWise EQMS (specific EQMS product) * ERP (Enterprise Resource Planning) systems * PLM (Product Lifecycle Management) systems * LIMS (Laboratory Information Management Systems) * CRM (Customer Relationship Management) systems * Document control systems Key Concepts: * **Enterprise Quality Management Software (EQMS):** A system designed to manage and automate quality processes across an entire enterprise, integrating data from various operational systems. * **Supply Chain Monitoring:** The process of tracking and overseeing the quality and performance of all stages and partners within a company's supply chain. * **Data Silos:** Disconnected data repositories within an organization that prevent information sharing and comprehensive analysis. * **Pillar Systems:** Foundational, critical enterprise systems like ERP, PLM, or LIMS that support core business functions. * **Quality Processes:** Standardized procedures and activities aimed at ensuring products or services meet specified quality standards. * **Deviation:** A departure from a standard procedure or specification. * **Customer Complaint:** An expression of dissatisfaction by a customer regarding a product or service. Examples/Case Studies: * **Manufacturing Deviation:** A part not fitting during the manufacturing process triggers a deviation record in EQMS. The system automatically pulls related data (serial number, lot number, supplier) from manufacturing, ERP, or product master data systems to facilitate rapid remediation. * **Customer Complaint:** A customer calls with a complaint about a missing or broken part. The customer service representative logs the complaint, and the EQMS integrates with CRM to get customer information and with ERP to retrieve product details (serial number, lot number), enabling quick triage and potential identification of existing issues for that specific lot.

Nasdaq Leads Mixed Session; Veeva, DoorDash, Urban Outfitters In Focus | Stock Market Today
Investor's Business Daily
/@investorsbusinessdaily
This video from Investor's Business Daily analyzes Thursday's stock market action, focusing on key movers and market trends. While primarily an investment-focused discussion, it highlights the performance and market attention on specific companies, including Veeva Systems, DoorDash, and Urban Outfitters, alongside broader market indices and the impact of major tech earnings. A significant theme is the continued and growing importance of AI in mega-cap tech companies and the chip sector. Key Takeaways: * **Veeva Systems' Market Prominence:** The video explicitly discusses Veeva Systems (referred to as "Viva Systems") as a stock "flirting with a breakout" and drawing significant attention from "growth investors" within the "software medical software group.". * **Sustained AI Investment Focus:** The hosts repeatedly emphasize that "AI an increasingly important part of a lot of the mega cap tech companies businesses" and that upcoming earnings will "still be all about AI, AI, AI.". * **Life Sciences Sector Activity:** The mention of "Edward Life Sciences, a medical products firms" gapping up after earnings indicates positive investor sentiment and activity within the broader medical and life sciences products sectorai that operate in niche, high-value sectors.

This OVERSOLD growth stock could have HUGE revenue in 2021 (30% upside potential)
Raylin Records
/@RaylinRecords
This.ai, and delves into the digital transformation occurring within the pharmaceutical and life sciences industries, particularly concerning clinical trials and operational efficiency. This video explores Veeva ($VEEV) as a leading cloud solution provider driving digitalization in the healthcare sector, specifically within the pharmaceutical and life sciences industries. The speaker discusses Veeva's financial performance and significant growth, highlighting its role in accelerating the shift towards digital, paperless, and patient-centric clinical trials. A major focus is placed on Veeva Vault Clinical applications and the groundbreaking Veeva e-consent, which enables electronic informed consent for clinical studies, streamlining processes and facilitating decentralized trials. The video emphasizes the vast market opportunity for digital healthcare solutions and the increasing adoption of cloud-based services accelerated by recent global events. Key Takeaways: * **Veeva's Central Role in Digital Healthcare:** Veeva is presented as a crucial cloud solution for healthcare, offering business consulting and training to major pharmaceutical clients like Merck, Moderna, and Eli Lilly, underscoring its deep integration into the industry. * **Pioneering Digital Consent and Decentralized Trials:** The introduction of Veeva e-consent marks a significant milestone, enabling the first fully digital, electronic informed consent in clinical trials. This innovation facilitates paperless, patient-centric studies and supports a "work from home study" model, allowing remote patient participation and real-time visibility for sponsors. * **Streamlining Clinical Operations:** Veeva Vault Clinical applications are highlighted for their ability to streamline global trial processes for Contract Research Organizations (CROs), enhancing efficiency, reducing administrative burden, and accelerating study execution. * **Massive Untapped Market Potential:** The digital healthcare community represents a substantial and growing total addressable market (TAM), estimated at over $12 billion. Veeva is positioned to capture a significant portion of this, with projections for substantial revenue growth across its diverse cloud services, including data cloud, patient data & analytics, and commercial cloud. * **Accelerated Digital Adoption Post-Pandemic:** The video notes that the pandemic has significantly accelerated the industry's move towards digital solutions, making cloud-based services essential for maintaining study timelines and improving overall trial execution. * **Focus on Efficiency and Patient Experience:** The core benefits of Veeva's innovations are increased efficiency, faster processes, reduced administrative tasks, and an improved patient experience by removing geographical and paper-based barriers in clinical research.

VEEVA & Salesforce Won't Tell You This: The AI Game Changer for Pharma Field Teams
Retorio
/@retorioofficial
This video from Retorio discusses the transformative impact of AI coaching on pharmaceutical field teams, particularly in overcoming challenges like digital channel saturation, underperforming drug launches, and the risk of losing direct HCP access to big tech platforms. It argues for a "renaissance of the field team," emphasizing the irreplaceable value of human connection and the need for sales reps and MSLs to possess both "warmth and competence" in their interactions with healthcare professionals. The video highlights how AI coaching can re-equip these teams, enabling them to practice and refine their messaging, empathy, and confidence in a safe, compliant, and scalable environment, ultimately leading to better commercial outcomes. Key Takeaways: * **AI Coaching for Commercial Excellence:** AI coaching is presented as a game-changer for pharma commercial and medical teams, transforming product lifecycles from pre-launch to maturity by enhancing human relationships and communication skills with HCPs. * **Addressing Digital Saturation & Underperformance:** Despite significant digital investments post-COVID, drug launches are underperforming due to oversaturated digital channels and a lack of meaningful human connection. AI coaching helps field teams cut through this noise by improving their direct engagement. * **Veeva/Salesforce & Data Ownership Risk:** The video cautions against over-reliance on big tech platforms like Veeva and Salesforce, suggesting they risk owning the entire value chain and potentially diminishing pharma companies' direct commercial capabilities and HCP relationships. * **Importance of "Warmth and Competence":** Effective HCP engagement requires field reps to demonstrate both warmth (empathy, active listening) and competence (applied knowledge, strategic communication). AI coaching provides targeted feedback to improve these critical behavioral dimensions. * **Compliant & Enterprise-Ready AI:** Solutions like Retorio emphasize enterprise readiness, EU AI Act compliance, data security, and MLR standards, ensuring that AI-powered coaching is safe, controlled, and free from hallucination risks, a crucial factor for the regulated pharma industry. * **Measurable Impact on Performance:** AI coaching delivers measurable results, including significant increases in field reps' warmth and competence scores, leading to higher customer satisfaction and faster product launch ramp-up periods (e.g., 38% faster launch ramp-up). * **Customizable AI Simulation:** The platform allows for the creation of customized coaching programs using a "Session Generator" (defining goals, integrating MLR-proof content) and a "Persona Generator" (creating digital twins of HCPs based on segmentation), enabling highly targeted and realistic role-play scenarios.

Future Of Veeva Vault Developer | Veeva Vault Developer Complete Guide | How to start learning Veeva
The Corporate Guys
/@TheCorporateGuys
This video provides an in-depth discussion on the future of Veeva Vault developers, detailing the platform's role as a specialized Enterprise Content Management (ECM) tool for the pharmaceutical industry. The speakers emphasize Veeva Vault's unique position due to its pharma-specific design, cloud-based accessibility, and comprehensive suites addressing critical areas like regulatory, clinical, and commercial operations. The conversation highlights the significant and sustained demand for Veeva Vault expertise, noting a current shortage of skilled professionals and projecting a bright future for those entering or advancing in this domain. Key insights include Veeva Vault's configuration-centric development approach, which allows individuals without extensive coding backgrounds to contribute, while also stressing the value of Java SDK, REST API, and VQL knowledge for advanced customization and integration. The discussion also touches upon the strategic importance of specific modules like Clinical Development Management System (CDMS), Electronic Trial Master File (eTMF), and Regulatory Submission, as well as the upcoming migration of Veeva CRM to Veeva Vault CRM, which is expected to create new opportunities for integrated solutions. Practical advice is offered on learning paths, emphasizing the need for hands-on experience within project environments due to the platform's non-open-source nature, and the importance of understanding core concepts like document versus object configuration. Key Takeaways: * **Veeva Vault's Pharma-Specific Dominance:** Veeva Vault is established as the premier cloud-based Enterprise Content Management (ECM) solution exclusively tailored for the pharmaceutical industry, facilitating migration from legacy systems and offering global accessibility. * **High Demand for Specialized Talent:** There is a robust and growing demand for Veeva Vault developers and configurators within the life sciences sector, significantly outpacing the current supply of skilled professionals, which translates into strong career prospects and financial growth. * **Configuration-Driven Development:** Veeva Vault development is largely configuration-based, making it accessible to individuals without deep coding expertise. However, proficiency in Java SDK, REST APIs, and VQL is highly advantageous for complex customizations, integrations, and automation. * **Comprehensive Industry Coverage:** The platform offers distinct product suites for critical pharmaceutical lifecycle domains, including Regulatory (e.g., Registration, Submission, Publish), Clinical (e.g., CDMS, eTMF), Commercial (e.g., PromoMats), and Quality Management Systems. * **Strategic Integration with Veeva CRM:** The planned migration of Veeva CRM to Veeva Vault CRM starting in 2025 represents a significant industry shift, promising enhanced integration capabilities and creating new opportunities for professionals skilled in both Veeva Vault and CRM. * **Learning and Skill Development:** Due to its proprietary nature, hands-on Veeva Vault experience is primarily gained through employer-provided access. Aspiring developers are advised to focus on understanding configuration differences (document vs. object), mastering Postman for API interactions, and leveraging official developer resources.

Bree Burks - Veeva
Note to File: A Clinical Research Podcast
/@notetofilepodcast
This video features Bree Burks from Veeva, discussing the company's significant advancements in clinical research technology. The conversation centers on Veeva's SiteVault, an e-regulatory system, and the highly anticipated launch of their new Clinical Trial Management System (CTMS) in August. A core theme is Veeva's strategy to bridge the gap between sponsors and research sites by fostering seamless, automated information exchange and creating a more unified, user-friendly technology experience for sites. The discussion highlights the industry's pervasive "information exchange problem" and the critical need for improved collaboration and consistent technological solutions to enhance clinical trial efficiency and patient focus, with a strong emphasis on the site's perspective and the challenges of change management. Key Takeaways: * **Veeva's Clinical Ecosystem Expansion:** Veeva is significantly expanding its clinical research offerings with a new CTMS, building upon its existing SiteVault e-regulatory system, aiming to create a more integrated platform for sites.ai specializes in Veeva CRM consulting and custom software development for the life sciences. * **Automated Data Exchange as a Game Changer:** Veeva's CTMS strategy focuses on automating data flow from sponsor/CRO systems (where Veeva has significant market saturation) directly into site systems, reducing manual re-entry and improving data consistency. * **Site Experience Drives Adoption:** The success of new clinical research technologies, including sponsor-site connections, is heavily dependent on a positive and consistent site user experience, which has historically been a barrier to adoption for previous initiatives like SIP. This highlights the importance of user-centric design in regulated environments. * **Change Management Overcomes Technology:** Implementing new technologies, especially those facilitating cross-organizational data exchange, faces significant hurdles in change management for both sponsors and sites, often more so than the technical development itself. This is a critical consideration for any firm offering AI and software solutions. * **Sites as a Shared Resource:** Sponsors are increasingly recognizing clinical research sites as a shared industry resource, necessitating collaborative efforts to provide consistent technology experiences across studies rather than disparate, sponsor-specific systems. * **Direct Communication is Key:** Initiatives like the Veeva Summit are crucial for fostering direct, transparent communication between sites and sponsors, enabling real-time feedback and collaborative problem-solving to drive industry-wide improvements. This emphasizes the value of community engagement in the life sciences tech space. * **Innovation Through Co-Development:** Veeva's approach to product development, particularly with the new CTMS, involves early release and co-development with customers to ensure the technology genuinely addresses site needs and scales effectively. This agile, customer-centric development model is a valuable insight for custom software development firms.

Accenture Interview Experience | Veeva Vault Developer Accenture Interview Process | Interview
The Corporate Guys
/@TheCorporateGuys
This video provides a detailed account of an Accenture interview experience for a Veeva Vault Developer position, focusing on the technical and HR rounds. The candidate, with 3.5 years of experience, was interviewed for a role specifically involving Veeva Commercial Vault, including PromoMats and MedComms. The technical interview delved into practical experience with configuration (life cycles, document workflows, objects), security models, jobs, flash reports, deployment processes, and handling general requests and incidents. It also covered integration experience. The subsequent managerial/HR round focused on career motivations and job location preferences. Key Takeaways: * **High Demand for Veeva Vault Expertise:** The detailed interview process underscores the critical need for skilled Veeva Vault developers in the life sciences sector, particularly within large consulting firms like Accenture. * **Emphasis on Practical Application:** Interview questions heavily focused on real-world scenarios, problem-solving methodologies (e.g., resolving issues, reducing service requests), and understanding of deployment and configuration processes. * **Core Technical Competencies:** Key technical areas evaluated included Veeva Vault's security model, jobs, flash reports, life cycles, document workflows, object configuration, and various integration types. * **Industry-Specific Content Knowledge:** Experience with specific Vault applications like PromoMats and and MedComms highlights the importance of understanding how Veeva Vault supports commercial and medical affairs content management in pharmaceuticals. * **Value of Support and Configuration Experience:** The interview probed both configuration/development and support-related experiences, indicating that a holistic understanding of Veeva Vault's operational lifecycle is highly valued.

Infosys Interview For 2 Years Experience || Veeva Vault Developer || Veeva Interview Experience
The Corporate Guys
/@TheCorporateGuys
This. Understanding the technical intricacies, configuration, and domain-specific applications of Veeva Vault, as detailed in the interview, isai in developing integrated solutions and providing expert consulting. This video details a candidate's interview experience for a Veeva Vault Developer position at Infosys, highlighting the technical and functional expertise required for such a role within the life sciences domain. The discussion covers the candidate's background working extensively with Veeva Vault in pharma and clinical projects, the multi-round interview process, and the specific types of questions encountered, which heavily focused on Veeva Vault configuration, security, and scenario-based problem-solving. Key Takeaways: * **Veeva Vault Technical Depth:** The interview questions underscore the necessity of deep technical knowledge in Veeva Vault, particularly regarding access control, lifecycle and workflow configurations, security permissions (profiles, licenses), custom objects, and administrative functions. * **Life Sciences Domain Expertise:** The candidate's experience and the interview's focus emphasize that practical application of Veeva Vault is predominantly within the life sciences (pharma, clinical), requiring domain-specific understanding for project execution, including migrations. * **Scenario-Based Problem Solving:** Interviewers heavily relied on scenario-based questions to assess practical problem-solving skills related to Veeva Vault functionalities (e.g., restricting user access, handling project deadlines, client interactions), indicating a need for applied knowledge beyond theoretical concepts. * **Agile and Client Management Skills:** The inclusion of questions on Agile methodology and client interaction highlights the importance of modern project management practices and soft skills for successful delivery in Veeva Vault projects. * **Pervasiveness of Veeva Vault:** The candidate's career progression within major IT service providers (Cognizant, Infosys) consistently working on Veeva Vault projects reinforces its widespread adoption and critical role in the pharmaceutical and life sciences sectors. * **Confidence from Knowledge:** The advice for aspiring candidates stresses that strong command over one's core technology and domain knowledge is the foundation for building confidence, which is crucial for effective interview performance.

Veeva CRM Online Training and Corporate Training by a professional trainer with Proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva CRM, positioning it as a specialized customer relationship management software tailored for the life sciences and pharmaceutical industries. It details how Veeva CRM helps pharmaceutical companies manage interactions with healthcare professionals (HCPs), track sales activities, and streamline sales and marketing processes. The discussion covers key benefits such as enhanced customer engagement, improved sales force effectiveness, robust compliance and regulatory support, and powerful analytics and reporting capabilities. The transcript delves into practical aspects like system setup, user roles, interface navigation, managing accounts and contacts, tracking activities, data capture best practices, and engaging with Key Opinion Leaders (KOLs). Furthermore, it highlights the importance of integrating Veeva CRM with other enterprise systems, advanced features like territory management and workflow configuration, and future trends including the impact of AI, machine learning, and predictive analytics on the platform. Key Takeaways: * **Veeva CRM's Strategic Role in Pharma Commercial Operations:** The video underscores Veeva CRM's critical function in optimizing sales and marketing within the pharmaceutical industry, extending beyond basic CRM to encompass compliance, HCP interaction tracking, and strategic KOL engagement * **Emphasis on Customization, Integration, and Data Quality:** The content highlights the flexibility of Veeva CRM through custom fields, forms, and workflows, alongside the necessity for seamless integration with other systems (CDPs, marketing automation, ERP).ai prioritizes in its AI-powered solutions for the regulated life sciences sector.ai to offer specialized AI/LLM solutions to enhance these strategic engagements. * **Future-Proofing with Emerging Technologies:** The discussion on future trends explicitly mentions the integration of AI, machine learning, and predictive analytics into Veeva CRM.

Veeva Vault Clinical eTMF Online Training: Comprehensive Guidelines | Proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva Vault Clinical eTMF, a modern solution for managing electronic Trial Master Files in clinical research. It delves into the crucial role of eTMF in maintaining compliance, ensuring data integrity, and streamlining trial processes. The discussion covers the benefits of digital TMFs, such as enhanced accessibility, reduced risk of document loss, and improved regulatory compliance, while also addressing associated challenges like data security, system integration, and user adoption. The video highlights Veeva Vault Clinical eTMF's features, including document management, workflow automation, electronic signatures, and audit trails, emphasizing its industry-specific expertise and configurability for efficient clinical trial management. Key Takeaways: * Veeva Vault Clinical eTMF serves as a cornerstone for modern clinical trial management, centralizing essential documents and significantly enhancing regulatory compliance and operational efficiency within the life sciences industry. * The transition to electronic TMFs offers critical advantages in accessibility, data integrity, and auditability, but necessitates robust strategies for data security, seamless system integration, and comprehensive user adoption to overcome inherent challenges. * Regulatory adherence, including GxP and audit trail requirements, is a primary driver for eTMF adoption, with Veeva Vault designed to meet these stringent industry standards crucial for pharmaceutical and biotech companies. * Successful implementation and ongoing management of eTMF platforms require comprehensive user training, strategic integration with other clinical trial systems, and collaboration with experienced vendors who understand regulatory requirements. * Veeva's deep industry knowledge and the configurability of its Vault platform are key differentiators, allowing organizations to tailor the solution to their specific workflows and processes within clinical operations and regulatory affairs. * The video implicitly underscores the broader need for sophisticated data engineering and integration solutions within the Veeva ecosystem, especially as Veeva transitions from its Salesforce foundation, presenting opportunities for specialized consulting.

Veeva Vault PromoMats Online Training: The Comprehensive Guideline | Proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva Vault PromoMats, positioning it as an indispensable, cloud-based content management solution tailored for the life sciences industry. It details how the platform streamlines the entire lifecycle of promotional materials, from collaborative creation and Medical, Legal, and Regulatory (MLR) review to multichannel distribution and electronic withdrawal of outdated content. A central theme is the platform's role in ensuring strict regulatory compliance, enhancing workflow efficiency, and fostering seamless collaboration among cross-functional teams in pharmaceutical, biotech, and medical device companies. The training outline covers core functionalities such as document creation, workflow automation, version control, digital asset management, and integration with other systems like CRM, all designed to reduce risks and accelerate time-to-market for compliant promotional content. Key Takeaways: * **Industry-Specific Content Management:** Veeva Vault PromoMats is a specialized, cutting-edge platform for managing promotional materials in the highly regulated pharmaceutical and life sciences sectors. * **Comprehensive Lifecycle Support:** It covers the full content lifecycle, including creation, MLR review, approval, distribution, tracking, and secure archiving, ensuring consistent messaging and compliance. * **Regulatory Compliance Focus:** The platform is built with compliance in mind, offering features like robust documentation, version control, audit trails, and controlled access to meet stringent regulatory requirements (e.g., FDA, EMA). * **Enhanced Efficiency and Collaboration:** PromoMats streamlines review and approval processes through automated workflows, significantly reducing manual errors, accelerating time-to-market, and improving collaboration across departments. * **Integration with Enterprise Systems:** It integrates seamlessly with other Veeva solutions and third-party systems, including CRM platforms, to ensure approved content accessibility for sales teams and efficient data exchange. * **Customization for Organizational Needs:** The platform allows for extensive customization of workspaces, templates, metadata, and review workflows to align with an organization's unique processes and branding. * **Strategic Importance and Career Opportunities:** The detailed training and discussion of career paths underscore the platform's critical role and widespread adoption within the life sciences industry, highlighting a demand for skilled professionals.

Live from Veeva Summit
Note to File: A Clinical Research Podcast
/@notetofilepodcast
Recorded live from the Veeva Summit, this video delves into critical discussions within the pharmaceutical and life sciences industries, specifically highlighting the evolving landscape of clinical research technology and stakeholder collaboration. The speakers discuss the Summit's emphasis on bringing together sponsors and sites to foster direct communication and problem-solving across the clinical trial lifecycle. A significant point of discussion is the launch of "Veeva AI," which aims to integrate data platforms with AI "agents," and the ongoing development of a full-fledged Clinical Trial Management System (CTMS). The conversation also touches upon the challenges of industry fragmentation and the need for standardization, as well as Veeva's increasing investment in solutions for clinical sites. Key Takeaways: * **Enhanced Sponsor-Site Collaboration:** The Veeva Summit actively promoted direct dialogue and collaboration between clinical trial sponsors and sites, identifying a shared understanding of challenges and a mutual desire to improve operational efficiencies throughout the protocol lifecycle. * **CROs as a Critical Missing Voice:** A notable observation was the underrepresentation of Contract Research Organizations (CROs) in these crucial sponsor-site discussions, suggesting a potential gap in comprehensive problem-solving that requires all key stakeholders at the table. * **Veeva's Strategic AI and CTMS Expansion:** Veeva announced the launch of "Veeva AI," signaling a paradigm shift towards integrating AI agents and data platforms, and is actively developing a comprehensive CTMS, indicating a significant push into AI-driven solutions and end-to-end clinical operations management. * **Industry Fragmentation and Standardization Challenges:** The speakers highlighted the pervasive issue of siloed operations and fragmentation within the clinical research ecosystem, even within sponsor organizations, underscoring the difficulty but necessity of establishing industry standards for increased productivity. * **Growing Investment in Site Solutions:** Veeva is significantly increasing its focus and investment in clinical sites, evidenced by a dedicated "site zone" at the Summit and a growing team, moving towards more holistic solutions beyond its initial electronic Investigator Site File (EISF) offering. * **Strategic Adoption of New Technologies:** For clinical sites, the adoption of new platforms like Veeva's evolving CTMS requires careful consideration of current capabilities and future roadmaps. It emphasizes the importance of providing feedback to influence product development and adopting solutions when they fully align with specific operational needs, such as integrated eSource.

Veeva Vault RIM: Comprehensive Online Training learn from experts with proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of a training program for Veeva Vault RIM, detailing its extensive functionalities for Regulatory Information Management within the pharmaceutical and life sciences industries. It covers the platform's role in streamlining compliance processes, managing regulatory documents and submissions (including IND, NDA, MAA), and ensuring regulatory success. The training curriculum explores key areas such as system configuration, user access control, document versioning, workflow automation, health authority communication, regulatory reporting, global alignment, and robust data integrity and security measures essential for compliance with standards like GxP and 21 CFR Part 11. The content underscores the platform's ability to enhance efficiency, collaboration, and reduce compliance risks across various regulatory activities Key Takeaways: * **End-to-End Regulatory Lifecycle Management:** Veeva Vault RIM offers comprehensive capabilities for managing the entire regulatory information lifecycle, from initial document authoring and submission planning to dossier management, publishing, and long-term archiving, which is crucial for pharmaceutical and life sciences companies. * **Enhanced Collaboration and Data Integration:** Veeva Vault RIM facilitates cross-functional collaboration, enabling efficient information sharing and integration of regulatory data with other critical departments such as Quality Assurance and Clinical Operations, streamlining overall operational workflows. * **Strategic Reporting and Global Alignment:** The system provides powerful reporting and analytics tools for tracking submission status, compliance trends, and key performance indicators. It also supports global regulatory alignment, helping organizations manage submissions and adhere to diverse international regulatory standards. * **Risk Management and Continuous Improvement:** Veeva Vault RIM supports proactive regulatory risk management through impact analysis for changes and fosters continuous improvement by identifying and addressing process inefficiencies, contributing to sustained regulatory excellence.

Capgemini Interview Experience | Veeva Vault Interview Questions 2025 | Capgemini Veeva Interview
The Corporate Guys
/@TheCorporateGuys
This video provides a detailed account of a Capgemini interview experience for a Veeva Vault Developer role, offering a comprehensive look into the technical and operational knowledge expected in this domain. The discussion covers a wide array of Veeva Vault functionalities, from security configurations and data management to workflow changes and regulatory information management (RIM). It also delves into support-related activities, system administration, and the importance of understanding Veeva's release cycles and compliance requirements.ai, which specializes in Veeva CRM consulting and AI solutions for the pharmaceutical and life sciences industries, understanding the technical depth and operational challenges associated with Veeva Vault is directly applicable to their service offerings, talent acquisition, and client solution strategies. Key Takeaways: * **Comprehensive Veeva Vault Expertise:** The interview questions demonstrate the need for a deep and broad understanding of Veeva Vault functionalities, encompassing security models (Atomic, DAC), data loaders, workflow configuration, and specific product modules like Regulatory Information Management (RIM). * **Strong Operational & Support Acumen:** A significant focus on daily activities, incident management, issue resolution processes (L1, L2, L3 support), and handling critical situations like system outages highlights the importance of operational excellence and robust support capabilities for Veeva Vault professionals. * **Regulatory Compliance Integration:** Questions concerning GxP applications, IQ OQ, and the nuances of RIM data (transactional, global, master) underscore Veeva Vault's critical role in maintaining regulatory adherence within the pharmaceutical and life sciences sectors. * **Veeva Product Lifecycle & Release Management:** The emphasis on Veeva General Releases, key features from recent updates (e.g., 24r1, 24r2), and processes for major releases indicates the necessity for professionals to stay current with Veeva's evolving platform and manage its deployment effectively. * **Advanced System Administration:** Interview topics covering user access management across multiple environments, Vault environment refresh procedures, and pre-release availability demonstrate the demand for skilled system administrators capable of complex Veeva Vault environment control.

Veeva Vault online training learn and improve your skill and Knowledge With Experts trainer
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva Vault, positioning it as a critical cloud-based content and data management solution specifically designed for the life sciences industry. The training covers fundamental aspects such as understanding Veeva Vault's purpose in streamlining processes, enhancing collaboration, and maintaining compliance. It delves into practical skills like navigating the platform, setting up user profiles and permissions, and mastering document management fundamentals including uploading, version control, and organizing files. The course also explores advanced functionalities such as collaborative workflows, customizing metadata, implementing robust data security and access controls, and utilizing advanced search capabilities. A significant portion is dedicated to regulatory compliance, electronic signatures, audit trails, workflow automation, and integration with other enterprise systems. The video further highlights Veeva Vault's applications in quality management, clinical trials, and regulatory submissions, underscoring its role in ensuring data integrity and adherence to industry standards. Key Takeaways: * **Veeva Vault as a Core Life Sciences Platform:** The video reinforces Veeva Vault's essential role as a document and data management system specifically designed for the life sciences, critical for managing regulated content across clinical, regulatory, and quality operations. * **Comprehensive Compliance & Data Integrity Features:** It details Veeva Vault's built-in capabilities for regulatory adherence, including electronic signatures, audit trails, version control, and robust access controls. * **Workflow Automation & Collaboration:** The platform's emphasis on configurable workflows, task assignment, and notification systems highlights its ability to automate routine tasks, streamline document review and approval processes, and enhance cross-functional collaboration.ai to integrate AI and LLM solutions for intelligent automation within these workflows. * **Data Engineering & Integration Potential:** The discussion on customizing metadata, advanced search, reporting/analytics, and API integration points to Veeva Vault's potential as a central hub for data.ai targets, such as clinical operations, medical affairs, and regulatory compliance.ai aims to solve.

Important Veeva Interview Questions | Veeva Vault Interview Questions 2025 | The Corporate Guys
The Corporate Guys
/@TheCorporateGuys
This video, presented by Vaibhav Agrawal from The Corporate Guys, delves into 15 important and recently asked interview questions pertaining to Veeva Vault. The discussion covers a broad spectrum of Veeva Vault functionalities, including how new general releases (like 24R3) are tested and assessed, the mechanics of Crosslink, and the critical role of Dynamic Access Control (DAC). The speaker also explains practical aspects such as retrieving deleted documents, various methods for extracting document and object metadata, and the utility of Flash Reports. Furthermore, the video touches upon fundamental Veeva concepts like Entry Criteria, Entry Actions, User Actions, different ways to create documents, types of field dependencies, Application Roles, and the distinctions between Document and Object Life Cycles. The content is geared towards professionals seeking to deepen their understanding of Veeva Vault and prepare for interviews in the life sciences sector, providing insights into both technical configurations and operational best practices. Key Takeaways: * **Veeva Release Management:** Understanding the process of testing and assessing new Veeva general releases (e.g., 24R3 features, auto-on vs. configurable features) is crucial for maintaining system integrity and compliance in a regulated environment. * **Core Veeva Functionalities:** Key concepts like Crosslink (for document creation and field value copying), Dynamic Access Control (DAC) for granular permissions, and User Role Setup are fundamental to Veeva Vault administration and security. * **Data Management & Retrieval:** Practical knowledge of retrieving deleted documents (within 30 days via Veeva support and API) and various methods for extracting metadata (Loader, UI export, API) is essential for operational efficiency and audit readiness. * **Reporting & Automation:** Flash Reports (scheduled reports) and their evolving capabilities (e.g., custom text in 24R3) offer powerful tools for automated communication and business intelligence within Veeva. * **Workflow & Configuration Basics:** A solid grasp of Entry Criteria, Entry Actions, User Actions, different document creation methods, and the three types of Field Dependencies (Field-based, Document Type-based, Document Life Cycle-based) is vital for effective Veeva Vault configuration and workflow design. * **Life Cycle Management:** Differentiating between Document and Object Life Cycles is a core aspect of managing content and data within Veeva Vault, directly impacting workflows and regulatory compliance.

Veeva Vault Interview Questions | Compliance Group Interview Experience | Veeva Interview Experience
The Corporate Guys
/@TheCorporateGuys
This video details a job interview experience for a Veeva Vault developer position at a company named "Compliance Group." The transcript outlines the multi-round recruitment process, focusing heavily on the technical interview questions for a candidate with six years of dedicated Veeva Vault experience. Key areas of questioning included Veeva Vault's security model, job scheduling and automation, general releases, deployment and migration best practices (including data packages and Vault Loaders), impact assessment, field-level security, workflows, life cycles, and object configuration. The managerial and HR rounds focused on career motivations and fit within a smaller, growing organization. Key Takeaways: * **Comprehensive Veeva Vault Technical Expertise:** The interview process demands in-depth knowledge of Veeva Vault's core functionalities, including security profiles, permission sets, user roles, job scheduling, automation tool integration, general releases, and intricate deployment and migration strategies. * **Criticality of Compliance and Best Practices:** The focus on "Compliance Group" and detailed questions about deployment considerations, migration impact assessments, and data integrity underscore the paramount importance of regulatory compliance and adherence to industry best practices in Veeva Vault implementations. * **Business Value Articulation:** Candidates are expected to not only possess technical skills but also to articulate the business use cases and benefits of the solutions they implement, such as automation tools integrated with Veeva Vault. * **Strategic Specialization in Regulated Tech:** The interviewee's career path highlights the value of deep, specialized experience in niche enterprise technologies like Veeva Vault within regulated industries, demonstrating a strategic move towards a compliance-focused role. * **Holistic Interview Preparation:** Beyond technical proficiency, success in such roles requires preparation for managerial questions concerning career motivations, organizational fit, and the ability to address challenges and lead teams, even in highly technical positions.

Veeva Vault Quality: The Complete Guide from experts with Proexcellency
Proexcellency Training
/@proexcellency_training
This video provides a comprehensive overview of Veeva Vault Quality, a cloud-based quality management system vital for highly regulated industries such as Pharmaceuticals and Life Sciences. It emphasizes how the platform streamlines quality processes, ensures regulatory compliance (including FDA, GxP, and ISO standards), and enhances operational efficiency. The content, framed as an online training course, delves into Veeva Vault Quality's architecture, core functionalities like document management, change control, risk management, and audit management, as well as its integration capabilities with other enterprise systems like ERPs. The discussion also highlights the benefits of flexible, tailored online training for professionals and briefly acknowledges the transformative role of technological innovations such as AI and IoT in modern quality management practices. Key Takeaways: * Veeva Vault Quality is presented as an essential, comprehensive cloud-based Quality Management System (QMS) specifically designed for the life sciences industry, addressing critical needs in pharmaceuticals, biotech, and medical devices. * The system offers robust functionalities for managing core quality processes, including document control, change control, risk assessment and mitigation, audit planning and tracking, and handling deviations and complaints. * Veeva Vault Quality supports integration with other enterprise systems, such as ERPs, to facilitate seamless data exchange and ensure data integrity across an organization's operational landscape. * The video briefly but significantly notes that technological innovations like Artificial Intelligence (AI) and the Internet of Things (IoT) are transforming quality management practices, indicating a future direction where AI solutions can augment platforms like Veeva Vault Quality. * The platform provides end-to-end visibility into quality processes, enabling data-driven decision-making, streamlining workflows, and fostering collaboration among cross-functional teams.

Veeva System Case Study || Veeva System Migration Specialist Interview Case Study || Veeva Vault Que
The Corporate Guys
/@TheCorporateGuys
This video presents a detailed case study for a Veeva Systems Migration Specialist interview, focusing on the practical execution of data and document migration to Veeva Vault Quality. The speaker, Vaibhav Agrawal, outlines a comprehensive migration strategy, including source data analysis, mapping document types, subtypes, classifications, and associated life cycles, as well as defining metadata fields and their dependencies. A significant portion of the discussion is dedicated to managing complex document versioning, particularly how to identify and migrate main versions while preserving historical versions within the Veeva Vault structure, often targeting a "Steady State Effective or Approved" status. The presentation also covers object creation with parent-child relationships using Veeva loader sheets and the importance of structuring a technical solution as a client-facing presentation. Key Takeaways: * **Veeva Vault Migration Process:** The video details a structured approach to Veeva Vault data migration, covering source data analysis, mapping strategies for document types and metadata, CSV preparation, and systematic data loading with error rectification. * **Complex Document Versioning:** A critical insight is the method for handling document versioning in Veeva Vault, which involves identifying main versions (e.g., major version >=1, minor version = 0) for initial upload, extracting document IDs, and then using these IDs to link and preserve minor versions within the version history tree. * **Object Relationship Management:** The case study highlights the importance of correctly identifying and establishing parent-child relationships between objects (e.g., Category and Sub-category) during migration, ensuring data integrity within Veeva. * **Veeva Loader Sheets & API/VQL:** The reliance on Veeva loader sheets for bulk data and document creation/update is a core technical takeaway, with a mention of API/VQL for advanced functionalities or data retrieval. * **Client-Centric Technical Presentation:** The speaker's advice to frame a technical interview case study as a client presentation offers a valuable perspective on how to communicate complex solutions effectively, focusing on process, strategy, and outcomes. * **Industry-Specific Context:** The discussion implicitly underscores the regulatory and operational rigor required in the pharmaceutical and life sciences industries, where precise document control, versioning, and data integrity within systems like Veeva Vault Quality are paramount.
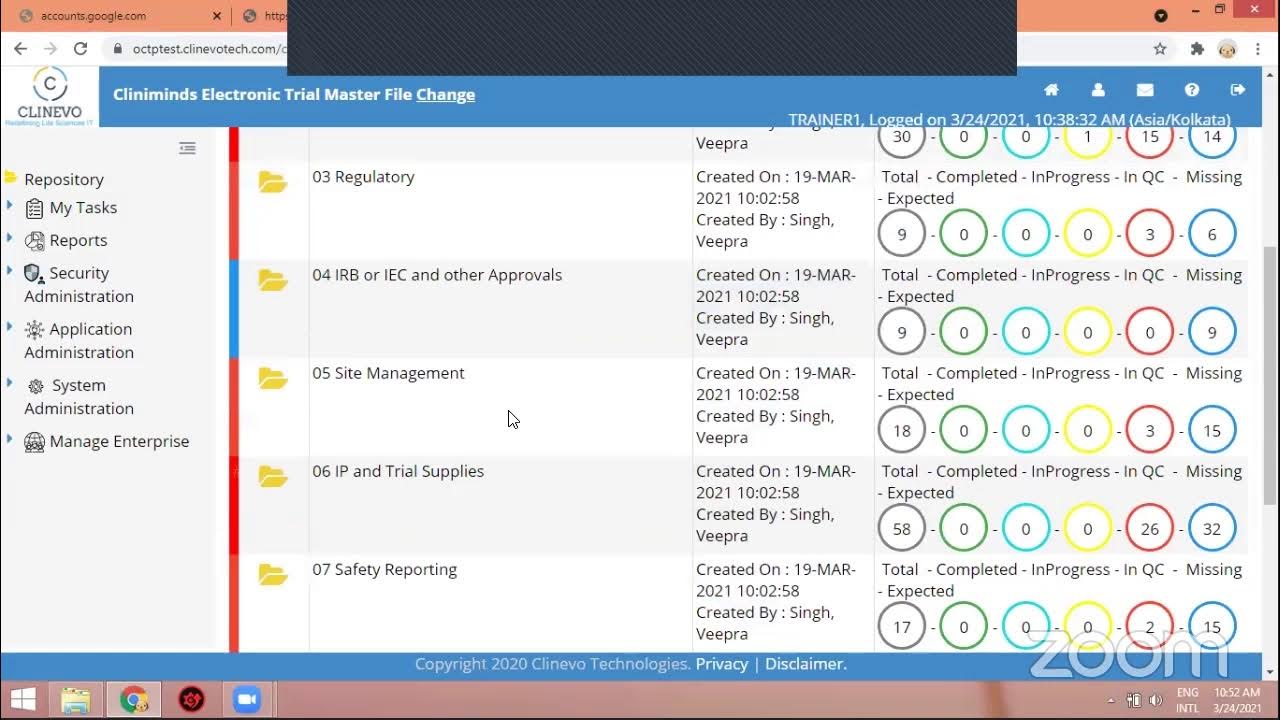
eTMF Software Hands on Practice - Cliniminds || Electronic Trial Master File
Cliniminds India
/@ClinimindsIndia
This video provides a hands-on, practical demonstration of utilizing an Electronic Trial Master File (eTMF) software system, focusing specifically on the critical workflow processes involved in managing clinical trial documentation. The primary purpose is to guide users through the step-by-step procedures for uploading, describing, and progressing essential documents like study protocols and case report forms (CRFs) through a regulated approval cycle. The tutorial emphasizes the importance of structured document management within a clinical research context, highlighting how an eTMF system facilitates organization, version control, and compliance. The demonstration begins with the fundamental action of uploading a document into a designated folder, illustrating how to use placeholders and context menus to initiate the upload process. It then delves into the crucial metadata associated with each document, such as providing a description, assigning a name, and setting workflow parameters like "auto-publish," along with effective and expiry dates. A significant portion of the video focuses on the document lifecycle, transitioning from an initial "open" status to subsequent stages like "pending for review" and "approved." This progression is managed through specific actions like "check-in," which is vital for version control and moving documents through the predefined workflow. Throughout the session, the instructor guides a participant through the software interface, troubleshooting minor operational issues like ensuring a document is selected before attempting a right-click action or saving a document before assigning reviewers. The core methodology demonstrated revolves around a role-based workflow, where documents move from an author to a reviewer and then an approver. This structured approach ensures that all necessary checks and balances are in place, aligning with regulatory requirements for clinical trial documentation. The practical examples of uploading a "protocol" and a "sample case report form" underscore the system's application to diverse clinical trial artifacts, reinforcing the necessity of meticulous documentation for regulatory adherence and audit readiness. Key Takeaways: * **eTMF as a Centralized Repository:** The video demonstrates an eTMF system as a crucial digital platform for organizing and managing all essential documents related to a clinical trial, ensuring a single source of truth for regulatory compliance and operational efficiency. * **Structured Document Upload Process:** Users are guided on how to upload documents using specific interface elements like placeholders, right-click menus, and "add document" options, emphasizing the systematic approach required within an eTMF. * **Importance of Document Metadata:** Critical information such as protocol description, document name, effective date, and expiry date must be accurately entered during upload. This metadata is essential for searchability, categorization, and compliance tracking. * **Automated Workflow Configuration:** The eTMF system allows for configuring workflows, such as "auto-publish," which dictates how documents progress through their lifecycle, minimizing manual intervention for routine tasks. * **Role-Based Document Review and Approval:** Clinical trial documents undergo a rigorous review and approval process involving different roles (e.g., author, reviewer, approver). The system facilitates assigning these roles to specific individuals to ensure accountability and compliance. * **Document Status Tracking:** The eTMF clearly displays the current status of each document (e.g., "open," "pending for review," "approved"), providing transparency and enabling real-time monitoring of documentation progress. * **"Check-in" for Version Control and Workflow Progression:** The "check-in" functionality is critical for formalizing changes, creating new versions, and moving a document to the next stage in its predefined workflow (e.g., from editing to review). * **Iterative Document Management:** The process of uploading, saving, reviewing, editing, and approving documents is iterative, often requiring multiple steps and interactions within the eTMF system to reach final approval. * **Handling Diverse Clinical Documents:** The demonstration covers the uploading of different types of essential clinical trial documents, specifically a "protocol" and a "case report form (CRF)," highlighting the system's versatility for various trial artifacts. * **Troubleshooting Common User Errors:** The tutorial implicitly addresses common user pitfalls, such as the necessity to first select a document before performing actions like right-clicking or ensuring a document is saved before attempting to assign reviewers. * **Regulatory Compliance Foundation:** The structured workflows, role assignments, and audit trails inherent in an eTMF system are foundational for meeting regulatory requirements from bodies like the FDA and EMA, ensuring GxP and 21 CFR Part 11 adherence. Key Concepts: * **eTMF (Electronic Trial Master File):** A digital system used to manage and store all essential documents for a clinical trial in a compliant and organized manner. * **Protocol:** A document that describes the objectives, design, methodology, statistical considerations, and organization of a clinical trial. * **Case Report Form (CRF):** A document, paper or electronic, designed to record all protocol-required information to be reported to the sponsor on each trial subject. * **Workflow:** A sequence of tasks or processes through which a document passes from initiation to completion, often involving multiple users and stages like review and approval. * **Auto-Publish:** A workflow setting that automatically publishes a document or moves it to the next stage upon meeting certain conditions, without manual intervention. * **Reviewer/Approver:** Roles assigned to individuals responsible for examining and formally sanctioning documents within the eTMF workflow. * **Check-in:** The action of saving a document back into the eTMF system, often creating a new version and potentially triggering the next step in its workflow. * **Document Status:** The current state of a document within its lifecycle (e.g., open, pending for review, approved, archived). Tools/Resources Mentioned: * An unspecified eTMF software system is demonstrated for hands-on practice.