Videos
Browse videos by topic
All Videos
Showing 1321-1344 of 1435 videos
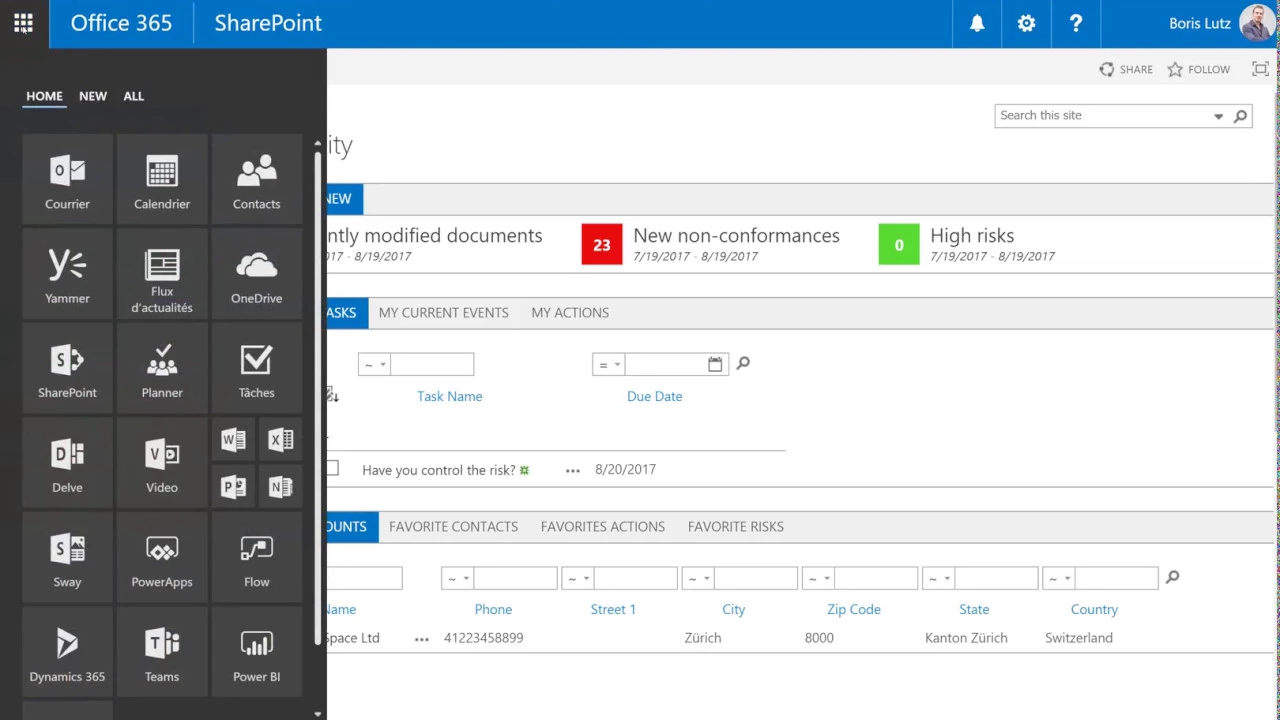
Quality and Risk Software for Microsoft Office 365
BPA Solutions
/@Bpa-solutionsNet
Aug 19, 2017
This video provides an in-depth exploration of BPA Quality 365, an all-in-one, interrelated quality and risk management software solution built on the Microsoft Office 365 platform. The presenter, Boris Lutz, CEO and founder at BPA Solutions, introduces the tool as designed to address common compliance standards, specifically highlighting ISO 9001:2015 and 21 CFR Part 11, making it suitable for regulated industries regardless of company size. The core purpose of BPA Quality is to foster a participative Quality Management System (QMS) by engaging all collaborators and facilitating continuous improvement through a user-friendly, integrated approach. The solution leverages the full suite of native Office 365 tools, including instant discussions, collaborative features, Microsoft Office applications, mobile solutions, workflow automation, and powerful reporting capabilities. It is designed with modular functions, allowing power users to customize and extend the solution without requiring any code. The system features role-based navigation, where displayed links are tailored to user permissions, covering areas like stakeholder management, process maps, strategic quality improvement, preventive risk management, compliance documents, best practices, and equipment management. A "What's New" cockpit keeps users informed of recent QMS changes, while manual and workflow tasks are prominently displayed on the home page. The video details several key modules and functionalities. The process map serves as the primary entry point for end-users, offering a 360-degree view of each process, including related compliance documents, risks with severity based on a risk matrix, improvement opportunities, KPIs, and best practices. An organizational chart allows drilling down into QMS processes grouped by responsibility, tracking associated collaborators, competencies, and training, thereby eliminating the need for manual spreadsheets. The non-conformance module enables internal or external users to declare issues, even via email, triggering a workflow to investigate, plan, and verify corrective actions (CAPA) before closure. A customizable non-conformance CAPA dashboard provides instant metrics without manual input. Mobile apps, built with PowerApps, allow remote declaration of non-conformances, photo capture, and geo-location, with automatic alerts and mobile decision-making. Microsoft Flow (now Power Automate) is used for building customizable workflows for resolution steps. The audit module facilitates planning audits in a shared calendar, inviting participants, selecting checklist questions, automatically generating reports, and declaring audit-related findings, actions, and risks. Finally, a dedicated risk module promotes a preventive attitude, showing the evolution of risk severity over time based on impact and probability, while document approval workflows drastically reduce the effort for document management and ensure collaborators are aware of changes. The system integrates with Microsoft Power BI for dynamic reporting, ultimately aiming for higher product and service quality with fewer errors. Key Takeaways: * **Integrated QMS and Risk Management:** BPA Quality 365 provides a comprehensive, all-in-one solution for managing quality and risk, ensuring that these critical aspects are interrelated and continuously improved. * **Regulatory Compliance Focus:** The software is specifically designed to meet stringent compliance standards, explicitly mentioning 21 CFR Part 11 and ISO 9001:2015, making it highly relevant for regulated industries like pharmaceuticals and life sciences. * **Leverages Microsoft Office 365 Ecosystem:** The solution is built natively on Office 365, enabling seamless integration with familiar tools for collaboration, mobile access, workflow automation, and reporting, enhancing user adoption and efficiency. * **Participative QMS Approach:** It promotes engagement from all collaborators, making the QMS participative rather than a top-down mandate, which can lead to better adherence and continuous improvement. * **No-Code Customization:** Power users can customize and extend the solution's modular functions without programming knowledge, allowing organizations to tailor it to their specific needs and evolving requirements. * **360-Degree Process View:** The process map offers a holistic view, linking processes to compliance documents, associated risks (with severity based on a risk matrix), improvement opportunities, KPIs, and best practices. * **Streamlined Non-Conformance and CAPA Management:** The system automates the entire non-conformance lifecycle, from declaration (including mobile and email inputs) through investigation, root cause analysis, CAPA planning, verification, and closure, providing instant metrics via a customizable dashboard. * **Mobile Capabilities for Field Operations:** PowerApps enable the creation of mobile applications for remote declaration of non-conformances, photo capture, geo-location, and mobile decision-making, improving real-time data collection and response. * **Proactive Risk Management:** A dedicated risk module supports a preventive attitude by allowing risk assessment, tracking the evolution of risk severity over time based on impact and probability, and helping avoid unfortunate events. * **Automated Document Control:** Document approval workflows significantly reduce the time and effort involved in managing document changes and approvals, ensuring that all collaborators are informed about new or updated documents. * **Efficient Audit Management:** The audit module facilitates planning, scheduling, checklist management, automatic report generation, and the declaration of findings, actions, and risks directly from the audit page, streamlining compliance verification. * **Powerful Business Intelligence and Reporting:** The solution integrates with Microsoft Power BI, allowing power users to build and share dynamic reports in minutes for any process, providing actionable insights for auditors and management. * **Elimination of Manual Processes:** The system replaces manual tracking methods, such as spreadsheets for responsibilities, competencies, and training, and automates dashboard updates, reducing human error and increasing efficiency. Tools/Resources Mentioned: * Microsoft Office 365 * Microsoft PowerApps * Microsoft Flow (now Power Automate) * Microsoft Power BI * ISO 9001:2015 * 21 CFR Part 11 Key Concepts: * **Quality Management System (QMS):** A formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. * **Risk Management:** The process of identifying, assessing, and controlling threats to an organization's capital and earnings. * **21 CFR Part 11:** Regulations issued by the FDA that set forth the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records and handwritten signatures. * **ISO 9001:2015:** An international standard for quality management systems, providing a framework for organizations to ensure consistent quality of products and services. * **CAPA (Corrective and Preventive Actions):** Actions taken to eliminate the causes of non-conformities or other undesirable situations to prevent recurrence (corrective) or occurrence (preventive). * **Non-conformance:** A deviation from a specification, standard, or expectation. * **Audit Management:** The systematic, independent, and documented process for obtaining audit evidence and evaluating it objectively to determine the extent to which audit criteria are fulfilled. * **Document Control:** The process of ensuring that all documents related to a QMS are properly managed, including creation, review, approval, distribution, and archiving. * **Process Map:** A visual representation of the steps involved in a process, showing the sequence of activities and decision points. * **Risk Matrix:** A tool used in risk assessment to define the level of risk by combining the likelihood of an event with the severity of its consequence.

Veeva Vault PromoMats Demo Video
Veeva Systems Inc
/@VeevaSystems
Aug 16, 2017
This video provides a detailed demonstration of the content lifecycle management process within the life sciences industry, utilizing Veeva Vault PromoMats as the central platform for digital asset management (DAM) and regulatory compliance. The scenario follows Janet, a global brand manager at Verdejo Biopharma, as she initiates a multi-channel campaign for the product Cola Cap. The process begins with Janet leveraging existing, approved assets from PromoMats to brief her creative agency. This establishes the platform's role in ensuring content consistency and global reuse from the outset, streamlining the initial creative phase and ensuring that all new materials are built upon a foundation of approved components, such as source image files and logos. The demonstration highlights the critical role of the Medical, Legal, and Regulatory (MLR) review process, emphasizing how Vault PromoMats accelerates approvals. Reviewers (like Carol) can access and comment on various digital assets—including eDetails, websites, and approved emails—in a responsive format across any device, wherever and whenever. This capability significantly speeds up the traditionally bottlenecked approval stage. Furthermore, the system facilitates global collaboration, as demonstrated when Janet requests an amendment from the creative agency (Joe); Joe is automatically notified and his team quickly makes required changes by leveraging source artwork directly from the materials proposed within Vault PromoMats, ensuring accuracy and compliance before final global approval. A significant portion of the workflow focuses on content localization and regional adoption. John, the regional brand manager for Europe, accesses Janet’s global toolkit, including campaign materials and links to approved claims and references, directly within PromoMats. Using the platform’s shopping cart feature, John selects appropriate components for the European market, quickly checking copyright status and making necessary local adjustments. The immediate ready access to source files, references, and claims simplifies the localization process, allowing John to efficiently assemble and share composite component and production files with local agencies, thereby reducing redundant work and accelerating time-to-market in specific regions like Spain. Finally, the video connects the approved content directly to commercial execution. Once localized and approved, the assets are automatically made available to the field force. Fredericka, a sales representative using Veeva CRM in Spain, utilizes the approved Cola Cap eDetail during a quick face-to-face meeting with a key opinion leader, Dr. Garcia. The successful interaction, including the doctor's engagement with the content and subsequent promise to review the associated campaign website, is tracked. This tracking data feeds back into the Vault PromoMats Digital Asset Dashboard, allowing both regional manager John and global manager Janet to measure the effectiveness and rapid adoption of the multi-channel campaign across various Spanish-speaking markets, demonstrating a closed-loop system from content creation to commercial insight. The system is explicitly designed to accelerate the digital supply chain for life sciences companies dealing with sophisticated multi-channel digital content. ### Key Takeaways: * **Centralized Digital Asset Management (DAM):** Veeva Vault PromoMats serves as the enterprise DAM system, enabling global brand managers to initiate new campaigns by locating and reusing existing, approved source materials (images, logos) to maintain brand consistency and compliance across all markets. * **Accelerated MLR Review:** The platform significantly speeds up the Medical, Legal, and Regulatory review process by allowing reviewers to access and comment on diverse digital assets (eDetails, websites, approved emails) in a responsive format on any device, eliminating traditional bottlenecks associated with manual review cycles. * **Compliance through Source Control:** When creative amendments are required, the agency leverages source artwork directly from the approved materials within Vault PromoMats, guaranteeing that all final assets are built upon a foundation of pre-approved, compliant components. * **Streamlined Localization:** Regional brand managers can efficiently adapt global assets for local markets using the PromoMats shopping cart feature, which provides immediate access to linked claims and references, simplifying the process of checking copyright and making necessary regional adjustments. * **Reduced Agency Workload:** By providing immediate access to source files, references, and claims, the system drastically reduces the amount of redundant work required by local agencies during the localization process, improving efficiency and accelerating time-to-market. * **Seamless CRM Integration:** Approved localized content is automatically made available to the field force through integration with Veeva CRM, ensuring that sales representatives are always using the most current, compliant materials (e.g., eDetails) during interactions with healthcare professionals (HCPs). * **Closed-Loop Commercial Insight:** The platform facilitates closed-loop marketing by tracking HCP engagement with the content presented via Veeva CRM (e.g., viewing an eDetail or promising to visit a campaign website), providing valuable activity data. * **Actionable Campaign Measurement:** The tracked activity data feeds into the Vault PromoMats Digital Asset Dashboard, allowing both global and regional managers to measure the success, effectiveness, and rapid adoption of multi-channel campaigns across various markets, enabling data-driven optimization. * **Digital Supply Chain Optimization:** The entire workflow is engineered to accelerate the digital supply chain for life sciences, managing complex multi-channel content from initial concept and global approval through localization, distribution, and final usage tracking in the field. ### Tools/Resources Mentioned: * **Veeva Vault PromoMats:** The primary platform demonstrated, used for enterprise Digital Asset Management (DAM), MLR review, content localization, and campaign tracking. * **Veeva CRM:** Used by the sales representative (Fredericka) in the field to present approved eDetails to healthcare professionals. * **Vault PromoMats Digital Asset Dashboard:** The business intelligence interface used by brand managers to track content adoption and measure campaign effectiveness. ### Key Concepts: * **Digital Asset Management (DAM):** The systematic organization, storage, and retrieval of digital assets (images, videos, documents) to ensure brand consistency and regulatory compliance. * **Medical, Legal, and Regulatory (MLR) Review:** The mandatory process in life sciences where all promotional and medical content must be reviewed and approved by internal experts to ensure adherence to regulatory standards (e.g., FDA, EMA). * **Content Localization:** The process of adapting global campaign materials (text, claims, references) to meet the specific cultural, linguistic, and regulatory requirements of a regional market. * **Digital Supply Chain (Life Sciences):** The end-to-end process of creating, reviewing, approving, distributing, and tracking compliant digital content for use across multiple channels (e.g., eDetail, website, email). ### Examples/Case Studies: * **Company:** Verdejo Biopharma (a fictional pharmaceutical company). * **Product:** Cola Cap (the product being promoted globally). * **Workflow:** The creation of a global campaign toolkit (landing page, leave-behind sheet, eDetail, approved email) initiated by Global Brand Manager Janet, reviewed by Carol (MLR), localized by Regional Brand Manager John (Europe), and executed by Sales Rep Fredericka (Spain).

Better Together - Veeva OpenData and Network
Veeva Systems Inc
/@VeevaSystems
Aug 12, 2017
This video provides a focused explanation of the synergy between Veeva OpenData and Veeva Network, detailing how their combined functionality addresses the persistent challenge of data decay and unreliable customer reference data within the pharmaceutical commercial landscape. The core premise is that sales representatives require accurate, current customer data—such as HCP and HCO information—to maintain productivity, engage effectively, and ensure regulatory compliance. The video establishes that traditional data management methods, characterized by slow vendor refreshes and complex loading processes, inevitably lead to "bad data," resulting in missed opportunities for meaningful customer engagement. Veeva OpenData is presented as the foundational solution, delivering continuous, leading-edge innovation for customer and compliance data. Its key differentiator is the proactive maintenance of data currency through real-time signaling across the industry’s largest CRM community. This approach moves beyond periodic updates, ensuring that customer data is always current and available precisely where it is needed: within the CRM system used by sales and marketing teams. A critical operational component highlighted is the role of expert data stewards who are committed to rapid inquiry resolution, with most issues being resolved in less than a day. This speed ensures that reps can quickly resume engagement activities while remaining compliant, supported by a simple, predictable pricing model that maximizes the value derived from the data asset. The video emphasizes that while OpenData provides the reliable data, managing this data using Veeva Network unlocks maximum efficiency. The integration of OpenData and Network eliminates the complex, time-consuming data maintenance routines that typically slow down business operations. By leveraging Network’s master data management capabilities alongside OpenData’s validated data feeds, life sciences companies gain the most reliable customer data that is not only always up-to-date but also instantly available within the CRM. This seamless integration translates directly into better-informed sales representatives, enabling them to drive more meaningful and compliant customer interactions, ultimately improving commercial effectiveness. Key Takeaways: • **Data Decay is a Commercial Liability:** The video stresses that outdated and untrustworthy customer reference data directly translates to missed opportunities for sales representatives to engage with customers in meaningful ways, hindering overall commercial productivity. • **Proactive, Real-Time Data Updates:** Veeva OpenData utilizes proactive updates and real-time signaling across the vast Veeva CRM community to ensure customer data remains current, moving beyond the limitations of slow, periodic data refreshes common with traditional vendors. • **Rapid Data Stewardship for Compliance:** Expert data stewards are central to the OpenData value proposition, resolving data inquiries quickly—most often in less than a day—which is crucial for enabling sales reps to maintain engagement momentum and stay compliant with regulatory requirements. • **OpenData and Network Synergy:** The optimal solution is the combined use of Veeva OpenData (the reliable data source) and Veeva Network (the master data management platform), which together eliminate the complex data maintenance routines that typically burden IT and commercial operations teams. • **Instant Data Availability in CRM:** The integration ensures that the most reliable, up-to-date customer data is instantly available within the CRM system, providing immediate value to sales and marketing teams without requiring manual loading or synchronization delays. • **Enhanced Sales Productivity and Compliance:** By providing reps with reliable, current data directly in their workflow, the solution drives better-informed interactions, leading to higher quality customer engagements and simplifying the process of maintaining regulatory adherence. • **Focus on Maximum Data Value:** The predictable pricing model and ongoing innovation associated with Veeva OpenData are designed to ensure maximum value is derived from the critical customer and compliance data assets. • **Elimination of Operational Drag:** Leveraging Veeva Network to manage OpenData removes the need for companies to manage complex data loading, cleansing, and synchronization processes, freeing up internal resources and accelerating business operations. • **Industry-Specific Data Community:** The solution benefits from the collective intelligence of the industry’s largest CRM community, which contributes to the accuracy and timeliness of the data through signaling and shared updates. Tools/Resources Mentioned: * Veeva OpenData * Veeva Network * Veeva CRM Key Concepts: * **Customer Reference Data:** The foundational information (names, addresses, specialties, affiliations) necessary for sales and marketing teams to identify and interact with healthcare professionals (HCPs) and healthcare organizations (HCOs). * **Real-Time Signaling:** A mechanism used across the Veeva community to instantly flag and update changes in customer data, ensuring high data currency and accuracy. * **Data Stewards:** Specialized personnel responsible for validating, correcting, and maintaining the quality and integrity of customer data within the OpenData system. * **Compliance Data:** Data specifically required to ensure that commercial interactions adhere to regulatory standards (e.g., FDA, EMA, Sunshine Act reporting), which is managed and kept current by OpenData.

Better Customer Data Means...Effective Compliance Activities
Veeva Systems Inc
@VeevaSystems
Aug 12, 2017
This video provides an in-depth exploration of the critical challenges faced by life sciences companies regarding aggregate spend reporting and regulatory compliance in an increasingly complex global marketplace. It introduces the scenario of Sanjay, a compliance officer at Virtio Biopharma, who is tasked with reconciling spend for practitioners across multiple countries to adhere to global transparency guidelines. The central theme established immediately is that the difficulty of reconciling disparate data sources from various internal systems directly compromises compliance efforts. The core problem highlighted is the significant risk associated with delivering inaccurate spend data to health authorities. This risk includes not only substantial financial penalties but also severe damage to the company’s reputation and credibility within the regulated industry. The complexity stems from the inability to uniquely identify healthcare professionals (HCPs) and organizations consistently across different systems and geographies, making accurate aggregation of associated spend nearly impossible using traditional methods. Sanjay’s primary need is a streamlined, reliable methodology for identifying practitioners and tracking all related financial disbursements. The solution presented is Veeva OpenData, which is positioned as a comprehensive master data management platform designed specifically to address these compliance and data quality issues. OpenData provides complete, accurate, and real-time customer data covering healthcare professionals, organizations, and stewardship services across all major global markets. By utilizing this unified, high-quality data source, compliance teams gain the ability to efficiently and accurately reconcile all spend data against uniquely identified customers. This unique identification capability is crucial for meeting the strict requirements of aggregate spend reporting regulations worldwide. The implementation of Veeva OpenData transforms the compliance workflow, enabling officers like Sanjay to confidently comply with diverse national and regional transparency requirements. The platform significantly reduces the operational burden traditionally associated with manually managing and reconciling fragmented spend data. The video concludes by emphasizing the value proposition of Veeva OpenData as an open, easy, and global solution for achieving data accuracy and regulatory confidence in the highly regulated pharmaceutical and life sciences sectors. ### Key Takeaways: * **Global Aggregate Spend Complexity:** Life sciences companies face major challenges in aggregating spend data across multiple international jurisdictions, driven by diverse and evolving national and regional transparency guidelines and regulations. * **Data Fragmentation as a Compliance Barrier:** The primary obstacle to accurate spend reporting is the inability to reconcile data originating from multiple, disparate internal systems, leading to inconsistencies and gaps in practitioner and organization identification. * **High Stakes of Inaccurate Reporting:** Submitting inaccurate aggregate spend data to health authorities carries considerable regulatory risk, including significant financial penalties and long-term damage to corporate reputation and trust. * **Necessity of Unified Customer Data:** Effective compliance requires a single source of truth for customer data (HCPs and organizations). This unified data must be accurate, complete, and available in real-time across all major operating countries. * **Veeva OpenData’s Role in Reconciliation:** Veeva OpenData is presented as the essential tool for overcoming data reconciliation difficulties by providing uniquely identified customer records, allowing compliance officers to accurately match spend transactions to the correct entities. * **Streamlining Regulatory Adherence:** Leveraging specialized master data platforms significantly reduces the manual burden of managing complex compliance data, allowing companies to efficiently meet national and regional transparency requirements with confidence. * **Data Stewardship Services:** The solution encompasses not just raw data but also stewardship services, ensuring the ongoing accuracy and maintenance of healthcare professional and organization records, which is vital for sustained compliance. * **Focus on Commercial Operations and Compliance Synergy:** The video underscores the interdependence of accurate commercial customer data (HCPs/organizations) and regulatory compliance, highlighting that data quality is foundational to regulatory risk mitigation. * **Actionable Data Strategy:** Companies must prioritize integrating master data solutions like Veeva OpenData into their existing enterprise architecture to ensure that spend data pipelines are robust, automated, and linked to verified customer identities. * **Mitigating Global Risk:** Adopting a "global, open, and easy" data solution is the strategic imperative for companies seeking to manage the increasing complexity of international transparency laws and ensure consistent adherence across all markets. ### Tools/Resources Mentioned: * **Veeva OpenData:** A data solution providing complete, accurate, and real-time customer data (HCPs and organizations) and stewardship services across major global countries, specifically designed to aid in aggregate spend reconciliation and compliance. ### Key Concepts: * **Aggregate Spend:** The total value of payments, transfers of value, or gifts provided by pharmaceutical or medical device companies to healthcare professionals (HCPs) and healthcare organizations (HCOs), which must be tracked and reported to regulatory bodies (e.g., under the U.S. Sunshine Act or similar global transparency laws). * **Transparency Guidelines and Regulations:** Mandatory rules (often enforced by bodies like the FDA or EMA) requiring life sciences companies to publicly disclose or report financial interactions with HCPs and HCOs to prevent conflicts of interest and ensure ethical practices. * **Practitioner/Customer Data Reconciliation:** The process of matching financial transactions from various internal systems against a definitive, verified list of healthcare professionals and organizations to ensure accurate reporting, often relying on unique identifiers. * **Health Authorities:** Government or regulatory bodies responsible for overseeing and enforcing compliance within the life sciences sector, such as the FDA (US) or national/regional equivalents.

Broadening Quality Management with Vault QMS at Atrium Innovations
Veeva Systems Inc
/@VeevaSystems
Aug 9, 2017
This video details Atrium Innovations' strategic expansion of their quality management system (QMS) using Veeva Vault, moving from static document control to integrated, action-level process management. James Huang, Director of Global Quality Systems, outlines how the company initially leveraged Vault QualityDocs for managing quality documents and GxP records across 14 global sites, establishing a single source of truth and robust authority control. The core value derived from this initial implementation centered on four primary areas: connected collaboration across sites, global transparent information sharing, secure storage and archiving, and immediate availability of documents during audits. This foundation set the stage for a significant milestone: the implementation of Vault QMS to handle event-level actions, change control, and overall workflow management. The transition to Vault QMS represents a shift from merely managing static information (documents) to actively managing events and actions within the quality lifecycle. This integrated approach allows Atrium to link tasks directly to individuals, track the status of quality events to conclusion, and enforce life cycle rules, thereby creating a more collaborative and unified system globally. A critical application of this expanded QMS is extending quality management beyond Atrium’s "four walls" to include third-party manufacturers and the entire supply chain. By integrating suppliers into the system for qualification, defect tracking, and change control, Atrium aims to improve quality across the entire supply chain, not just internally. Furthermore, the implementation of Vault QMS is leveraged as a powerful workload management tool, a functionality not originally anticipated but highly valued. By utilizing the system's dashboard capabilities, Atrium gains transparency into process progress, eliminating the ambiguity common with older, folder-based systems where progress claims were difficult to verify. This transparency allows management to track the movement of documents and tasks, ensuring accountability and driving processes forward. Atrium Innovations is also implementing Vault for registration and submission modules alongside QMS, emphasizing the power of a "connected and unified" Veeva ecosystem where the entire product lifecycle—from application submission to documentation—is seamlessly linked. Key Takeaways: • **Transition from Static to Action-Oriented QMS:** Companies should evolve their quality systems beyond simple document storage (like QualityDocs) to event-level management (like QMS) to handle change control, CAPAs, and deviations, ensuring tasks are linked to specific individuals and followed through their complete lifecycle. • **Supply Chain Quality Integration:** Modern QMS implementation must extend beyond internal operations to include third-party manufacturers and suppliers. Integrating suppliers into the system allows for unified qualification processes, defect tracking, and collaborative change control, fostering quality improvement across the entire supply chain. • **Four Pillars of Global QMS Value:** The primary benefits of a unified global QMS system are: 1) Connected collaboration across all sites; 2) Global, transparent information sharing; 3) Secure, worry-free storage and archiving; and 4) Immediate availability of information for audits and regulatory inquiries. • **QMS as a Workload Management Tool:** Beyond its core regulatory function, a modern QMS platform can be leveraged for workload management and transparency. Dashboards and status tracking eliminate the "no transparency" issues of legacy systems, allowing managers to monitor task progress and ensure accountability globally. • **Harmonization through Technology:** Implementing a unified technology platform like Veeva Vault provides a critical opportunity to harmonize disparate business processes across different global sites, ensuring consistency in quality operations and regulatory adherence. • **Importance of a Unified Ecosystem:** Implementing QMS alongside other modules, such as Registration and Submission, creates a powerful "connected and unified" ecosystem. This integration ensures that quality events and documentation are seamlessly linked to regulatory submissions and product applications, providing end-to-end visibility. • **Audit Readiness and Availability:** A key value proposition of cloud-based, controlled document management systems is the immediate availability of required information during audits, significantly streamlining the compliance verification process. • **Change Control Integration:** The ability to link change control processes directly to specific tasks and individuals within the QMS is crucial for maintaining compliance and ensuring that changes are thoroughly reviewed, approved, and implemented across all involved parties (internal and external). Tools/Resources Mentioned: * Veeva Vault QualityDocs * Veeva Vault QMS (Quality Management System) * Veeva Vault Registration and Submission modules Key Concepts: * **Global QMS Implementation:** The process of deploying a single, standardized Quality Management System across multiple international sites to ensure process harmonization and compliance consistency. * **Connected and Unified System:** The concept of integrating various functional modules (e.g., Quality, Regulatory, Submissions) within a single platform (Veeva Vault) to ensure seamless data flow and end-to-end process visibility. * **Event-Level Management:** Moving beyond static document control to actively manage quality events (e.g., deviations, CAPAs, change controls) through defined workflows, task assignments, and lifecycle tracking.

Standardizing on Vault QualityDocs Globally at Johnson & Johnson
Veeva Systems Inc
/@VeevaSystems
Aug 9, 2017
This video provides an in-depth case study on the global deployment and standardization of Veeva Vault QualityDocs across Johnson & Johnson (J&J), as presented by Garrett Sayers, Senior Manager of Quality Systems and Shared Services. The primary objective of the initiative was to replace a complex, fragmented legacy technology landscape with a unified, cloud-based solution that could handle the scale and stringent regulatory requirements of a massive global enterprise. J&J successfully rolled out the system to 60 countries, managing over 15 languages, highlighting the inherent agility and efficiency of modern cloud solutions compared to older, legacy applications. A core strategic decision underpinning the successful deployment was the commitment to minimizing customization. J&J deliberately aimed to leverage as much "off-the-shelf" or "out-of-the-box" functionality as possible, setting an internal target of 15% configuration. While the final configuration level reached approximately 18%, this disciplined approach prevented scope creep and ensured a faster, more standardized rollout. This strategy also contributed significantly to reduced support overhead; the team observed a substantial decrease in demand for support services, user tickets, and queries compared to their legacy systems, demonstrating the system's stability and improved user experience. The transition to Vault QualityDocs delivered profound operational improvements, particularly in simplifying global document management processes crucial for GxP compliance. Sayers shared a powerful anecdote illustrating the previous inefficiencies: business units were literally FedExing physical documents between distant locations, such as Korea and Cambodia, for signatures. The implementation of the cloud-based system eliminated this manual, time-consuming process, allowing authorized personnel to digitally sign off on documents instantly, regardless of their physical location or time zone. Ultimately, the standardization effort served to modernize document management and successfully integrate previously underserved or siloed business units globally, ensuring operational consistency and bringing these teams into the future of compliant document control. Key Takeaways: * **Cloud Agility for Global Scale:** Utilizing a cloud solution like Veeva Vault QualityDocs enabled J&J to achieve a rapid and efficient global rollout across 60 countries and 15+ languages, demonstrating the platform's ability to handle massive, complex organizational structures inherent to the life sciences sector. * **Prioritize Out-of-the-Box Functionality:** The most critical success factor was the strict adherence to minimizing customization; J&J aimed for 15% configuration and achieved 18%, emphasizing that leveraging standard features accelerates deployment, simplifies validation, and reduces long-term maintenance complexity. * **Reduced Support Burden:** The transition away from older, complex applications to the more dynamic Vault QualityDocs resulted in a noticeable decrease in demand for support services, user tickets, and queries, indicating improved system stability and a better overall user experience that requires less intervention. * **Standardization Drives Efficiency:** Standardizing on a single quality document management system eliminated fragmented processes and replaced highly inefficient manual workflows, such as the need to physically ship documents (e.g., via FedEx) across continents for required signatures. * **Modernizing Regulatory Compliance:** The system facilitates immediate digital sign-offs, which is crucial for maintaining GxP compliance and audit readiness, replacing archaic methods with modern, traceable, and time-zone-agnostic processes vital for global operations. * **Strategic Configuration Targets:** Setting a clear, low configuration target (e.g., 15%) is a vital best practice for large-scale enterprise deployments, helping project teams resist pressure for unnecessary customizations that complicate future system upgrades and regulatory validation cycles. * **Inclusion of Previously Siloed Teams:** The global rollout successfully brought previously neglected or marginalized business units into a modern, standardized document management framework, ensuring consistency and future-proofing operations across the entire enterprise. * **Dynamic System vs. Legacy Applications:** The speaker noted that Vault QualityDocs is "more dynamic" than older applications in use across J&J, suggesting that modern, specialized SaaS solutions offer superior flexibility, integration capabilities, and user experience compared to legacy enterprise systems. * **Addressing Time Zone Management:** The system inherently solves the challenge of managing time zones and expectations across global teams by providing 24/7 access and instant digital sign-off capabilities, a necessity for a company operating in 60 countries. * **User Delight and Adoption:** The ability for users to log on and sign documents instantly, even while traveling, significantly improved the daily life and efficiency of business users, which is key to driving high adoption rates in regulated software systems. Tools/Resources Mentioned: * Veeva Vault QualityDocs * FedEx (mentioned as the legacy method for document transfer) Key Concepts: * **Off-the-Shelf/Out-of-the-Box Functionality:** The strategy of using a software system with minimal changes to its default settings and features, which is critical for maintaining ease of upgrades and standardization in regulated GxP environments. * **Global Standardization:** The process of unifying disparate systems and processes across multiple countries and regions (60 countries, 15+ languages) to achieve operational consistency and centralized control over quality documentation. * **Configuration vs. Customization:** J&J’s approach clearly distinguishes between *configuration* (setting up the system using built-in tools, targeted at 15%) and *customization* (writing new code or modifying the core software structure), which was actively avoided to maintain regulatory compliance and system integrity.

Optimizing Global Content Reuse at Shire
Veeva Systems Inc
/@VeevaSystems
Aug 9, 2017
This presentation outlines Shire’s strategic vision for optimizing the global content lifecycle, moving from initial concept development through regulatory approval, production, and eventual customer use. The core objective is to establish a unified, efficient, and compliant platform that streamlines the creation and dissemination of both promotional and non-promotional materials across international markets. The speaker emphasizes the necessity of integrating a complete feedback loop, particularly for digital sales aids, to track usage metrics—such as which pages or messages are utilized by representatives (Reps) and Medical Science Liaisons (MSLs)—and feeding that data back to central marketing teams in Zurich. This data-driven approach is designed to continuously refine content and messaging, ultimately enabling healthcare professionals (HCPs) to make informed prescribing decisions. The initiative focuses heavily on consolidating disparate, inefficient legacy systems—including paper-based processes, different SharePoint sites, email approvals, and proprietary systems like Zinc—into a single, global platform, specifically mentioning the migration to "PromoMats" (Veeva PromoMats) and a centralized Digital Asset Management (DAM) system. This consolidation is projected to yield significant efficiencies, allowing for global approval processes managed by a centralized Commercial, Medical, and Regulatory (CMLR) team. The goal is to dramatically reduce the time-to-market for essential assets, including global press releases and website content, by leveraging native files stored securely on the DAM. A key performance indicator for this transformation is the increase in global content reuse. The speaker notes that historical reuse figures were low (around 10-11%) and sets an ambitious target of achieving 30% to 40% reuse of core international materials. Furthermore, the project aims to drastically cut the approval cycle time, moving from an average of 40 days down to an optimal 7 to 8 days. Strategically, the company is shifting from a "push" model—where global teams manually disseminate content, creating high workload for local countries—to a "pull" system. This allows local affiliates to access core, compliant assets directly from the DAM, providing the necessary foundation for local customization while ensuring regulatory adherence. The primary challenge identified in this complex migration is securing comprehensive stakeholder engagement, particularly among senior leadership, IT, and CMLR business partners, to ensure smooth transition and adoption of the new unified platform. Key Takeaways: • **End-to-End Content Lifecycle Optimization:** The vision encompasses the entire content journey, from concept development and CMLR review/approval through production, dissemination, and post-usage feedback, linking marketing strategy directly to field execution. • **Data-Driven Feedback Loops:** Essential for digital sales aids, the system must capture usage metrics (e.g., which pages were viewed, how messages were used) and feed this data back to marketing to refine content and improve message effectiveness for HCPs. • **Consolidation of Legacy Systems:** A major efficiency driver is the migration away from fragmented systems (paper, email, SharePoint, Zinc) to a single, global platform (Veeva PromoMats/DAM) to standardize the review and approval process internationally. • **Global CMLR Standardization:** Establishing a global CMLR team and platform enables unified approval of core global assets (e.g., press releases, websites), significantly improving regulatory compliance and reducing time-to-market. • **Aggressive Efficiency Targets:** The company aims to reduce the content approval timeline from an average of 40 days to a target of 7 to 8 days, demonstrating a focus on speed and agility in commercial operations. • **Targeted Content Reuse Metrics:** The strategic goal is to increase the global reuse of compliant core materials from a low baseline (10-11%) to an ambitious 30% to 40%, maximizing investment in content creation and minimizing agency costs. • **Shift from "Push" to "Pull" Dissemination:** Moving to a pull system allows local countries to access and retrieve assets directly from the DAM, reducing global dissemination workload while allowing local teams the flexibility to adapt materials based on specific country requirements. • **Compliance and Compelling Content:** The core mandate is to produce material that is both compelling for the audience and strictly compliant with regulatory standards, ensuring the integrity of the information shared with HCPs. • **Stakeholder Engagement is Critical:** The most significant challenge in migrating assets and implementing the new system is ensuring robust engagement and buy-in from all key stakeholders, including senior leadership, IT, and regulatory/business partners, from the project's inception. • **Key Metrics for Success:** Success is measured across multiple points: the number of cycles required for approval, the time taken to reach production, content usage metrics by the field force, and the final content reuse percentage internationally. Tools/Resources Mentioned: * **Veeva PromoMats (implied by "promo man"):** The platform used for managing promotional and non-promotional content review, approval, and distribution. * **Digital Asset Management (DAM):** The centralized repository for storing native files and approved assets for global access and reuse. * **Digital Sales Aid:** A specific content format used by representatives (Reps) and MSLs in the field, providing measurable usage data. * **Zinc:** A legacy system mentioned as a source of assets being migrated. Key Concepts: * **CMLR (Commercial, Medical, Legal, Regulatory):** The cross-functional team responsible for reviewing and approving all pharmaceutical content to ensure regulatory compliance before distribution. * **Push vs. Pull System:** A "push" system involves global teams actively sending content to local markets; a "pull" system allows local markets to retrieve (pull) content on demand from the central DAM. * **Time-to-Market:** A critical metric measuring the duration from content concept development to its final approved use by the field force.

United Therapeutics' Best Practices for Upgrading from Zinc MAPS to Vault PromoMats
Veeva Systems Inc
/@VeevaSystems
Aug 9, 2017
This video provides an in-depth look at the strategic decision-making and operational best practices employed by United Therapeutics during their migration from the legacy Zinc MAPS platform to Veeva Vault PromoMats. The primary impetus for this transition was regulatory compliance, driven by the company's compliance team who sought to be on the most current and future-proof platform available, recognizing that Zinc MAPS would eventually be phased out. United Therapeutics positioned itself as an early adopter to proactively manage this critical infrastructure shift, ensuring continuous adherence to industry standards for promotional material review and approval. While compliance was the initial strategic driver, the migration unlocked significant operational and commercial benefits that were not originally anticipated. A key advantage identified is the integrated Digital Asset Management (DAM) system native to Vault PromoMats. This integration eliminates the need for United Therapeutics to rely on a separate, third-party cloud-based storage solution for managing digital assets, streamlining the content lifecycle and reducing system complexity. Furthermore, the move facilitates seamless integration with other existing Veeva channels already deployed within the organization, adding a new dimension of connectivity and workflow automation that was previously unattainable with the older Zinc platform. The speaker emphasizes that the enhanced reporting capabilities within Veeva Vault PromoMats offer substantial added value compared to the limited reporting available in Zinc MAPS. These superior business intelligence tools allow for deeper insights into the content review process, cycle times, and asset utilization. Crucially, the success of the migration was tied not just to technical execution, but to meticulous planning, particularly concerning organizational readiness and communication. The core advice centers on three critical preparatory phases essential for a smooth transition. These include ensuring all contractual and licensing agreements are finalized before any technical work begins; rigorously reviewing and finalizing all user settings and workflows to ensure they meet current and future business needs; and proactively educating the implementation team on the wider range of services and functionalities available within the comprehensive Veeva ecosystem, maximizing the return on investment in the new platform. --- ## Key Takeaways: * **Compliance as the Primary Migration Driver:** The decision to move from Zinc MAPS to Veeva Vault PromoMats was fundamentally driven by the compliance team's mandate to operate on the latest regulatory platform, anticipating the eventual end-of-life for the legacy system. Proactive migration ensures long-term regulatory stability. * **Integrated DAM is a Major Operational Gain:** A significant, non-compliance benefit of Vault PromoMats is its integrated Digital Asset Management (DAM) system, which replaces the need for separate, third-party cloud-based storage solutions, simplifying IT architecture and improving asset accessibility. * **Maximize Ecosystem Integration:** The migration facilitates seamless integration with other existing Veeva channels already deployed across the enterprise. Organizations should leverage this capability to create end-to-end workflows that were previously impossible with non-integrated systems like Zinc MAPS. * **Prioritize Change Management Communications:** Successful adoption hinges on robust change management. Organizations must meticulously plan communications to all new users, detailing planned changes, timelines, and promptly communicating any delays to maintain user support and buy-in throughout the process. * **Secure Contracts and Licenses Upfront:** Before initiating any technical migration work, it is critical to finalize all necessary contractual elements, including the Scope of Works for the standard migration and securing all required user licenses. Completing this administrative work early prevents costly delays. * **Pre-Migration Workflow Validation:** A crucial best practice is to thoroughly check and finalize all user settings and workflows *before* the migration begins. This ensures the new system is configured correctly to meet business requirements from day one, avoiding mid-migration adjustments. * **Leverage Enhanced Reporting Capabilities:** Veeva Vault PromoMats offers superior reporting capabilities compared to Zinc MAPS. Implementation teams should focus on utilizing these advanced features to gain actionable business intelligence regarding content review cycles and commercial operations efficiency. * **Familiarize with the Broader Veeva Ecosystem:** Implementation teams and stakeholders should familiarize themselves with the full range of services available within the Veeva platform, as it extends far beyond the functionalities offered by Zinc MAPS, allowing the organization to maximize its investment in the new technology. --- ## Tools/Resources Mentioned: * **Zinc MAPS:** The legacy platform for promotional material review and approval that United Therapeutics migrated away from. * **Veeva Vault PromoMats:** The target platform, a cloud-based application for managing the end-to-end promotional materials process, including Medical, Legal, and Regulatory (MLR) review, and Digital Asset Management (DAM). * **Veeva Channels:** Refers to other applications within the Veeva ecosystem (e.g., Veeva CRM, Veeva Vault Clinical, etc.) that can integrate with PromoMats. ## Key Concepts: * **Digital Asset Management (DAM):** A system for organizing, storing, and retrieving rich media assets. The integration of DAM within Vault PromoMats is highlighted as a major benefit, simplifying asset governance within the regulated environment. * **Compliance Platform:** Refers to software designed to manage regulated processes (like MLR review) and ensure adherence to standards set by bodies like the FDA and EMA. The migration was driven by the need to be on the "latest compliance platform." * **Scope of Works (SOW):** A formal document detailing the work activities, deliverables, and timeline for the migration project, emphasized as needing to be finalized before the project commences. ## Examples/Case Studies: * **United Therapeutics:** The pharmaceutical/biotech company whose migration experience from Zinc MAPS to Veeva Vault PromoMats serves as the central case study, illustrating the strategic drivers (compliance) and operational benefits (integrated DAM, enhanced reporting) of the transition.

Accelerating Go-to-market Strategies with Integrated Customer Data
Veeva Systems Inc
@VeevaSystems
Aug 9, 2017
This video provides an in-depth exploration of accelerating pharmaceutical go-to-market strategies through the rapid implementation and integration of core commercial solutions, specifically focusing on the Veeva ecosystem. The speaker recounts the challenge of deploying all necessary commercial platforms—including CRM, Patient Services, Approved Email, and regulated content management—within a stringent six-month timeline and constrained budget. The foundational strategy adopted was to maintain simplicity, maximize the use of standard, out-of-the-box functionality provided by Veeva, and focus strictly on essential requirements to ensure timely launch readiness. A critical operational and regulatory challenge discussed was the need to comply with HIPAA requirements, which strictly prohibit sharing patient data between different organizational functions. This necessitated the creation of two separate CRM environments: one for commercial operations and one for patient services. The solution to maintaining data consistency and efficiency across these firewalled systems was the integration of both CRM environments with a single Master Data Management (MDM) system, Veeva Network. This integration successfully maintained the required regulatory separation between commercial and patient services functions while leveraging a unified, accurate source of master data. The speaker highlights the significant value achieved through the seamless integration of Veeva CRM and Veeva Network. Traditionally, sales representatives were tasked with manually entering and updating every piece of information about a healthcare professional (HCP), including affiliations, location changes, and retirement status. By integrating Network, the responsibility for maintaining the accuracy of this foundational customer data was shifted away from the sales force. Automated data flows ensured that updates—such as an HCP moving or changing their name—were instantly reflected in the CRM, freeing sales reps from administrative burdens and allowing them to concentrate on core commercial activities. The core advice offered to other companies preparing for a rapid product launch is to adopt an accelerated, focused approach. This involves clearly defining the core essentials needed for launch, securing immediate buy-in from the customer groups for this expedited strategy, and recognizing Veeva as the "best of breed" solution for commercial CRM and MDM. By leveraging the platform's robust out-of-the-box capabilities and utilizing available professional services, the team successfully closed critical operational gaps, achieving a highly successful implementation within the tight budget and timeframe, exceeding internal expectations. Key Takeaways: • **Prioritize Simplicity for Accelerated Launches:** When faced with tight timelines and limited resources for a commercial launch, the most effective strategy is to keep the implementation simple, focusing only on core essentials and leveraging standard, out-of-the-box functionality from platform providers like Veeva. • **Veeva as the Commercial Ecosystem Standard:** The speaker strongly advocates for Veeva as the "best of breed" solution in the commercial space, specifically for Master Data Management (MDM), CRM, and the management of regulated content (e.g., Approved Email), suggesting reliance on their established foundation. • **Regulatory Compliance Mandates System Separation:** Strict regulatory requirements, such as HIPAA, often necessitate the creation of separate, firewalled CRM environments to prevent the sharing of sensitive patient data between commercial functions and patient services functions. • **MDM Unification Across Segregated Systems:** Even when regulatory constraints require multiple CRM environments (e.g., commercial and patient services), a single, integrated Master Data Management system (like Veeva Network) can be used to connect both environments, ensuring data accuracy while maintaining the necessary regulatory firewall. • **Automated Data Management Drives Commercial Efficiency:** Integrating CRM with an MDM solution like Veeva Network eliminates the administrative burden on sales representatives to manually track and update HCP data (e.g., moves, retirements, name changes, affiliations). This automation ensures higher data quality and allows the sales force to focus purely on customer engagement. • **Strategic Use of Professional Services:** For rapid, complex implementations, leveraging the professional services offered by the platform vendor (Veeva) is crucial for closing operational gaps quickly and efficiently within constrained timeframes and budgets. • **Secure Customer Buy-in for Accelerated Timelines:** Success in a rapid implementation requires securing explicit buy-in from the internal customer groups (commercial operations, sales) that an accelerated, potentially less customized, approach will be taken. • **Focus on Core Commercial Solutions:** The successful rapid implementation covered core solutions including CRM, Patient Services, Approved Email, and Regulated Content, highlighting the essential technology stack required for a modern pharmaceutical commercial launch. Tools/Resources Mentioned: * **Veeva CRM:** The core customer relationship management platform. * **Veeva Network:** The Master Data Management (MDM) system used for integrating and maintaining accurate customer data (HCPs, affiliations). * **Veeva Patient Services:** A specialized solution designed to manage patient support functions while adhering to regulatory requirements. * **Veeva Approved Email/Regulated Content:** Solutions for managing and distributing compliant, regulated communications. Key Concepts: * **Master Data Management (MDM):** A comprehensive method of defining and managing the critical non-transactional data of an organization, ensuring a single, accurate source of truth for customer data (HCPs). * **HIPAA Requirements:** The regulatory standards in the US governing the protection of sensitive patient health information, which often dictates the separation of data environments within pharmaceutical companies. * **Go-to-market Strategy:** The plan detailing how a company will deliver its product or service to reach target customers and achieve a competitive advantage, often centered around a product launch. * **Out-of-the-Box Approach:** The strategy of using software solutions with minimal customization, relying on the standard functionality provided by the vendor to accelerate deployment and reduce complexity.

Maximizing Your CRM Investment
Veeva Systems Inc
@VeevaSystems
Aug 9, 2017
This video outlines LEO Pharma's strategic framework for maximizing its investment in Veeva CRM by systematically integrating innovation adoption into its operational lifecycle. The core strategy revolves around focusing on specific Veeva releases that deliver tangible value and align directly with the company's overarching business objectives. LEO Pharma emphasizes that staying current with the latest Veeva innovations is not an ad-hoc process but is systematically built into their application maintenance life cycle, allowing them to consistently "sense the opportunities," "seize the relevant opportunities," and then "transform the business" to meet strategic goals. The presentation highlights three key Veeva CRM innovations that have been successfully implemented: Approved Email, Closed Loop Marketing (CLM), and Consent Capture. These features were prioritized because they enable LEO Pharma to engage with their customers (healthcare professionals) in a much more direct, personal, and tailored manner, ensuring communications are relevant to the target audience. The successful integration of these tools relies heavily on a robust governance structure, which includes two distinct advisory boards: the System Advisory Board, which is responsible for setting the high-level strategic direction for the entire Veeva application, and the Change Advisory Board (CAB), which operates on the operational level. The CAB assesses and approves every change request before it moves into the development, testing, and production implementation phases. A critical component of maximizing investment and ensuring ROI is achieving high user adoption. LEO Pharma addresses this through a structured training methodology centered on "super user training camps." When large changes are implemented—such as the rollout of Approved Email and CLM—super users from global affiliates are gathered, trained extensively on the new functionality, and then tasked with returning to their local markets to train the end-users. This decentralized, expert-led training model ensures consistency and effective knowledge transfer across the organization. The speaker concludes by summarizing the three best practices for maximizing CRM investment: ensuring the right competencies are available in the right places, integrating release management activities into the core fabric of daily operations, and establishing rigorous governance structures. Key Takeaways: • **Systematic Innovation Adoption:** LEO Pharma treats the adoption of new Veeva features as a structured process built into the application maintenance life cycle, moving systematically from sensing opportunities to seizing them, and finally transforming the business to align with strategic goals. • **Dual-Layered Governance Model:** Effective CRM management requires two distinct governance bodies: a System Advisory Board (SAB) for setting strategic direction and a Change Advisory Board (CAB) for operational approval, ensuring changes are both strategically sound and operationally feasible. • **Prioritizing Customer Engagement Features:** The top implemented innovations—Approved Email, CLM, and Consent Capture—were selected specifically because they facilitate direct, personalized, and tailored engagement with customers, enhancing the quality and relevance of commercial interactions. • **Integration of Release Management:** A core best practice is ensuring that release management activities are not treated as separate projects but are fully integrated into the "fabric" of daily operations, making continuous improvement a standard operating procedure. • **Competency Management is Crucial:** Maximizing CRM investment depends fundamentally on ensuring that the right technical and operational competencies are secured and placed strategically within the organization to manage and leverage the platform effectively. • **Super User Training Camps for Global Adoption:** To drive user adoption, especially for major global changes (like CLM), LEO Pharma utilizes centralized super user training camps, empowering local experts to cascade knowledge and training to end-users in their respective affiliates. • **Operational Change Vetting:** The Change Advisory Board (CAB) plays a vital role in assessing every incoming change request, ensuring thorough vetting before development, testing, and production implementation occurs, thereby maintaining system stability and compliance. • **Strategic Alignment is Paramount:** The fundamental goal of adopting new Veeva features is to ensure they bring value to the business and actively support the overarching commercial and organizational strategy, preventing the implementation of non-essential features. Tools/Resources Mentioned: * **Veeva CRM:** The core platform being discussed. * **Approved Email (Veeva):** Feature used for compliant, personalized email communication with healthcare professionals (HCPs). * **Closed Loop Marketing (CLM) (Veeva):** Feature used to track and optimize digital content interactions during sales calls. * **Consent Capture (Veeva):** Feature used to compliantly manage and record customer consent preferences. Key Concepts: * **Application Maintenance Life Cycle:** The systematic process LEO Pharma uses to manage, update, and improve its Veeva CRM application, incorporating new releases and innovations. * **System Advisory Board (SAB):** A high-level governance body responsible for defining the long-term strategic direction and vision for the Veeva application within the organization. * **Change Advisory Board (CAB):** An operational governance body responsible for reviewing, assessing, and approving specific change requests before they are implemented into the production environment. * **Super User Training Camps:** A methodology for training key personnel (super users) globally on major new functionalities, who then become local trainers responsible for cascading knowledge to end-users.

Getting the Most out of Veeva CRM: Best Practices for Release Governance
Veeva Systems Inc
/@VeevaSystems
Aug 9, 2017
This video provides an in-depth exploration of best practices for Veeva CRM release governance, focusing on how Teva Pharmaceuticals successfully leveraged structured release management to maximize platform adoption and fundamentally improve the relationship between their IT department and commercial business units. The core objective articulated by the IT leadership is achieving high adoption and utilization rates in the field by delivering a tool that is both easy to use and fully exploits the native capabilities of the Veeva platform, thereby minimizing the need for complex, costly customizations. A critical component of Teva’s strategy involves a rigorous, proactive approach to evaluating new features included in every Veeva CRM release. Rather than waiting for the business to request specific functionality, the IT team, in collaboration with their managed services group and the Veeva Customer Success Manager (CSM), prototypes and implements optional features in a controlled environment. This allows them to assess the potential value of these new capabilities and present them to the business stakeholders, ensuring that the organization capitalizes on continuous platform improvements without incurring significant development overhead. The most profound outcome of their refined release management process has been the introduction of transparency, which has transformed the dynamic between IT and the commercial business. By openly communicating how resources are utilized and integrating the business into the governance structure, IT has moved from being a service provider to a strategic partner. This partnership is formalized through a joint steering committee. This committee is responsible for assessing all new projects, functionalities, and enhancements that impact the Veeva ecosystem. This shared decision-making process ensures that all proposed changes are vetted to confirm they will provide tangible benefits to the field force while minimizing disruption to existing commercial operations. By laying this groundwork and ensuring the business is part of the decision-making process, Teva has significantly improved the overall quality and effectiveness of their Veeva CRM investment. Key Takeaways: • **Adoption and Utilization are the Primary Success Metrics:** The ultimate measure of success for Veeva CRM implementation is not technical stability, but rather achieving high adoption and utilization rates among the field force, which directly correlates to commercial effectiveness. • **Prioritize Native Functionality:** To maximize ROI and simplify future upgrades, organizations should focus on exploiting all available capabilities within the core Veeva platform, actively avoiding unnecessary customization that can complicate maintenance and release cycles. • **Proactive Feature Evaluation is Essential:** Organizations should establish a formal, structured process to evaluate every new feature released by Veeva (typically three times per year) to ensure they are capitalizing on platform evolution and continuous improvements. • **Leverage External Expertise for Prototyping:** Best practice involves utilizing the Veeva Customer Success Manager (CSM) and managed services partners to efficiently implement and prototype optional features in a sandbox environment, streamlining the internal assessment process. • **Mandatory Business Value Assessment:** Before any new feature or enhancement is deployed, IT must collaborate with the business to clearly define the value proposition and ensure the change will genuinely benefit the field force and align with commercial objectives. • **Change Management Underpins Success:** Robust change management practices are critical for successfully introducing new features and enhancements, ensuring that the field force is prepared, trained, and able to integrate the changes smoothly, thereby sustaining high utilization rates. • **Transparency Fosters Partnership:** Introducing transparency into the release management process—specifically detailing resource allocation and project timelines—is key to building trust and transforming the relationship between IT and the commercial business into a strategic partnership. • **Implement a Joint Steering Committee:** A governance best practice is the formation of a joint steering committee composed of both IT and business leaders to collectively assess and approve all new projects, enhancements, and functionalities impacting the Veeva platform. • **Shared Decision-Making Minimizes Resistance:** Integrating business stakeholders into the decision-making process for Veeva enhancements ensures shared ownership, minimizes resistance to change, and guarantees that technological updates are driven by genuine commercial needs. • **Focus on Minimal Field Disruption:** All proposed functionalities and enhancements must be rigorously vetted by the steering committee to ensure the impact on the day-to-day workflow of the field force is minimal, thereby maintaining productivity and avoiding user frustration. Tools/Resources Mentioned: * Veeva CRM * Managed Services Group * Customer Success Manager (CSM) Key Concepts: * **Release Governance:** The structured process and policies used to manage the introduction of new features, updates, and changes to the Veeva CRM platform, ensuring stability and alignment with business goals. * **Change Management Practices:** Methodologies used to prepare, support, and equip individuals to successfully adopt change, crucial for maintaining high utilization rates following a Veeva release. * **IT-Business Partnership:** A collaborative model where the IT department acts as a strategic partner to the commercial business, sharing decision-making authority and ensuring technology investments directly support commercial outcomes. Examples/Case Studies: * **Teva Pharmaceuticals:** The video uses Teva Pharmaceuticals as a case study, demonstrating how their implementation of structured release management and governance processes successfully improved IT-business relationships and maximized Veeva CRM adoption.

Rapidly Deploying a Global Quality Solution at Shire
Veeva Systems Inc
@VeevaSystems
Aug 9, 2017
This video details Shire’s experience in the rapid, global deployment of Veeva Vault QualityDocs, a specialized document management system (DMS), following the company’s divestiture from its parent organization. The core challenge was the immediate need to establish a compliant, standalone quality solution capable of managing approximately 1.2 million legacy documents and supporting 20,000 users across the world. Anil R. Bharwani, Director of IT, Quality and Environmental, Health, and Safety at Shire, explains that their previous system, a Product Lifecycle Management (PLM) solution, was fundamentally inadequate for the specific requirements of pure document management, necessitating a comprehensive search for a specialized replacement. The vendor selection process was rigorous, starting with 18 potential companies before narrowing down to a few finalists. Veeva Vault QualityDocs was evaluated against other top industry vendors and ultimately chosen due to its superior user experience and functionality. Key benefits cited by Shire included the system’s intuitiveness, ease of use (requiring very little training), highly effective search capabilities, and robust collaboration features, all of which significantly surpassed the capabilities of their previously used internal systems. The project was executed under an extremely tight deadline. Funding was secured in January, and Phase One of the global deployment went live in July, making the new system immediately accessible to all 20,000 global users within six months. The immediate success was quantifiable, with post-go-live statistics showing phenomenal activity, including over 400,000 sign-ins and 200,000 document changes recorded shortly after launch. This rapid adoption validated the system's user-centric design and the effectiveness of the deployment strategy. A critical piece of actionable advice offered by the Shire team, based on their experience migrating massive amounts of data, is the absolute necessity of dedicating time to map and clean legacy data structures before moving them into Veeva Vault. This proactive data hygiene step is crucial for minimizing backend issues and ensuring a smooth transition for end-users. Key Takeaways: • **Veeva Vault as a Critical Divestiture Solution:** The project was driven by the need for a standalone, compliant IT infrastructure following a corporate separation. Veeva Vault QualityDocs proved capable of handling the massive scale (1.2 million documents, 20,000 users) and the aggressive timeline required for rapid operational independence in a regulated environment. • **Superiority of Specialized DMS:** Shire’s evaluation process, which included 18 initial vendors, confirmed that specialized platforms like Veeva QualityDocs offer significant advantages over generalized enterprise systems (like their previous PLM system) when it comes to core document management, collaboration, and search functionality in a GxP context. • **Aggressive Deployment Benchmarks:** The successful transition from project funding approval in January to global Phase One go-live in July sets a critical benchmark for rapid enterprise software deployment within the pharmaceutical sector, demonstrating that large-scale, compliant system implementations can be achieved within six months. • **User Experience Drives Adoption:** The primary benefits highlighted—easy to use, intuitive, and requiring very little training—underscore that user-centric design is paramount for achieving high user adoption rates, especially when rolling out a new regulated system to tens of thousands of global users. • **Data Preparation is the Single Most Important Step:** The most crucial recommendation from the implementation team is to invest significant effort in mapping and cleaning legacy data before migration. Failure to clean source data will lead to substantial issues on the backend and negatively impact user satisfaction once the data resides in Veeva Vault. • **Validation Through Usage Metrics:** Immediate and high-volume usage statistics, such as over 400,000 sign-ins and 200,000 document changes shortly after launch, serve as concrete evidence of successful system adoption and immediate operational value realization. • **Addressing PLM Limitations:** The speaker explicitly noted that while their previous PLM system performed certain tasks well, it struggled with the specific demands of pure document management. This insight is valuable for consultants advising clients to move specialized functions, like Quality Docs, onto dedicated platforms. • **Focus on Collaboration and Search:** Beyond basic storage, the enhanced collaboration features and intuitive search capabilities of Veeva QualityDocs were key differentiators that improved operational efficiency compared to Shire's previous system. Tools/Resources Mentioned: * **Veeva Vault QualityDocs:** The implemented document management system (DMS) tailored for quality and regulatory documents. * **PLM (Product Lifecycle Management) System:** The legacy system previously used for document management, which was deemed inadequate for the scale and specific needs of a global quality solution. Key Concepts: * **Legacy Data Mapping:** The essential process of defining the structure, metadata, and relationships of data from the old system to ensure accurate and compliant transfer into the new Veeva Vault environment. * **Global Quality Solution:** A unified, standardized system for managing quality documentation and processes across all worldwide sites, crucial for maintaining consistent regulatory compliance (implied GxP adherence). Examples/Case Studies: * **Shire Implementation:** A case study demonstrating rapid, large-scale migration and deployment, involving 1.2 million documents and 20,000 users globally, executed within a six-month window.

Atrium Innovations: Benefits of Unifying Quality in the Cloud
Veeva Systems Inc
@VeevaSystems
Aug 9, 2017
This video provides an in-depth exploration of the strategic benefits realized by Atrium Innovations through the implementation of a unified, cloud-based Quality Management System (QMS), likely leveraging Veeva’s industry-specific platform. The central theme articulated by James Huang is the critical necessity of moving beyond fragmented, localized quality systems to a single, connected, and globally consistent platform. This unification is presented not just as a technological upgrade, but as a foundational shift required for global life sciences companies to maintain operational efficiency and stringent regulatory compliance. A major focus of the discussion is the challenge of information fragmentation. The speaker highlights the common problem of having "information silos" or "studs of information" scattered across various systems and locations. The unified cloud approach solves this by creating a "global transfer system" where critical quality data and documentation are instantly available across the entire organization. This connectivity ensures that information is readily accessible "at fingertips," eliminating delays and risks associated with relying on outdated or localized data stores. For a global company, this immediate availability of accurate, controlled information is paramount for rapid decision-making and audit readiness. Furthermore, the presentation emphasizes the role of the unified platform in streamlining complex operational workflows. The speaker notes the importance of robust "workflow management" to handle quality processes—such as deviations, CAPA, and change control—which often become bottlenecks in disparate systems. By integrating these processes, the company gains "cruise control" over its quality lifecycle, suggesting a high degree of automation and standardization. This integration is achieved by connecting and unifying various "Veeva modieus" (modules), allowing the organization to achieve "real progress" by ensuring that quality processes are consistently executed from the initial stages, such as documentation created in the lab, through final review and implementation. Ultimately, the goal of Atrium Innovations’ transition was to connect and unify the entire quality work stream across all global sites. This holistic approach ensures that every part of the organization, from R&D to commercial operations, adheres to the same quality standards and uses the same validated documentation. This strategic move is vital for maintaining GxP compliance and ensuring that the company’s quality posture is robust and auditable worldwide, reinforcing the value proposition of specialized, cloud-based solutions tailored for regulated industries. Key Takeaways: * **Mandate for Quality Unification:** The primary strategic benefit discussed is the move toward a single, unified quality system in the cloud, replacing disparate legacy systems to achieve global connectivity and control over critical processes. * **Elimination of Information Silos:** A unified platform is essential for breaking down "information silos," ensuring that quality documentation and data are not localized but are instantly accessible and consistent across all global sites. * **Global Consistency in Documentation:** Implementing a "global transfer system" ensures that all standard operating procedures (SOPs), batch records, and quality documents are standardized and managed centrally, which is crucial for multi-national regulatory compliance. * **Enhanced Workflow Management:** The unified system provides robust tools for "workflow management," allowing the organization to streamline complex quality processes (e.g., investigations, testing, approvals) and reduce manual intervention. * **Achieving Process "Cruise Control":** Standardization and automation within the unified system enable organizations to gain better control over their quality lifecycle, leading to more predictable outcomes and faster cycle times for critical quality events. * **Integration of Veeva Modules:** The successful implementation relies on integrating various Veeva modules (implied, likely QualityOne or QualityDocs) to ensure that documentation, training, and process management are seamlessly connected within a single ecosystem. * **Data Accessibility for Compliance:** Immediate access to accurate, controlled information "at fingertips" significantly enhances audit readiness and facilitates rapid responses to regulatory inquiries by providing a single source of truth. * **Connecting the Entire Quality Chain:** The solution is designed to connect the entire quality process, starting from the initial data generation (e.g., "talking de labo") through to final commercial implementation, ensuring end-to-end quality oversight. * **Enabling Real Progress:** By unifying and automating quality processes, the company can shift focus from managing administrative overhead to driving genuine quality improvements and innovation. Tools/Resources Mentioned: * Veeva Systems (Implied, as the channel and context point to Veeva’s Quality Management solutions like QualityOne or QualityDocs). * Cloud-based Quality Management System (QMS). Key Concepts: * **Unified Quality in the Cloud:** The strategy of consolidating all quality management processes, documentation, and data onto a single, validated cloud platform accessible globally. * **Global Transfer System:** A centralized system designed to manage and distribute quality-critical information and documentation consistently across all international operating units. * **Workflow Management:** The automated control and tracking of quality processes (e.g., change control, deviations, CAPA) within the QMS to ensure adherence to defined procedures and regulatory requirements.

Leveraging Insights to Tailor the HCP Experience
Veeva Systems Inc
@VeevaSystems
Aug 9, 2017
This video outlines a strategic shift for pharmaceutical companies toward customer-centric engagement by leveraging data and technology to tailor the Healthcare Professional (HCP) experience. The core message emphasizes moving beyond traditional, outbound marketing objectives to focus instead on content that adds genuine value and resonates with the customer's needs and interests. This requires a fundamental change in mentality, prioritizing what the customer wants to hear over what the company wants to say, thereby ensuring that content consumption—which often has marketing objectives—is perceived as valuable by the recipient. Adopting this customer-first approach is presented as a foundational path to adding significant value to a pharmaceutical company's operations. A foundational element of this strategy is the implementation and sophisticated utilization of a robust technology platform, specifically Veeva CRM. The speaker stresses that the platform must evolve beyond its traditional function as a simple tracking tool—merely counting how many times a field representative has interacted with a customer. Instead, the goal is to use Veeva as a powerful insights tool. This transformation allows the organization to analyze which specific messaging, delivered across various channels, is most effective and resonant with the target customer base. By establishing a strong, harmonized technological foundation across all operating markets, the company can streamline operations, freeing up resources to concentrate much more heavily on strategic planning, channel optimization, performance measurement, and continuous improvement thereafter. The most critical strategic concept discussed is the identification and targeting of "moments that matter" in the customer journey. The speaker challenges commercial teams to critically understand the specific contexts and moments when a customer (HCP or patient) is most receptive to information or requires support. The strategy dictates that if a company can understand precisely where its customers are and what moments are important and relevant to them, then the company can ensure its own relevance in those moments. Examples provided include the critical moment when a patient leaves a waiting room after being diagnosed with a specific disease, or when a customer has a complex question that cannot be answered simply or immediately. Identifying these high-value moments and placing the commercial strategy accordingly is presented as the key to maximizing engagement and impact. Key Takeaways: * **Value-Driven Content Strategy:** Pharmaceutical companies must transition their content creation from fulfilling internal marketing objectives (what the company wants to say) to providing information that genuinely adds value and addresses the specific needs of the customer base (what the customer wants to hear). * **Veeva CRM as an Insights Engine:** The foundational technology platform, explicitly named as Veeva, must be utilized beyond its traditional function as a transactional CRM used for counting field rep interactions; it must be leveraged as a sophisticated tool for generating actionable customer insights. * **Measure Message Resonance Across Channels:** A primary use of the CRM platform is to analyze data to determine precisely which specific messaging is resonating most effectively and which communication channels are yielding the highest engagement rates within the target customer base. * **Focus on Strategy through Technology Harmonization:** Establishing a great foundational technology across all operating markets allows the commercial team to shift their focus away from infrastructure management and toward higher-value activities like strategic planning, channel optimization, and performance measurement. * **Identify Critical "Moments That Matter":** Commercial strategy must center on critically understanding and identifying the specific moments in the customer journey where information or support is most relevant, timely, and impactful. * **Strategic Relevance through Timeliness:** Relevance is achieved by placing resources and communication strategies precisely at these identified critical moments. Examples include providing support immediately following a patient diagnosis or offering complex, detailed answers when an HCP faces a difficult clinical question. * **Continuous Improvement Cycle:** The engagement process is cyclical, requiring continuous measurement and improvement. Teams must define the strategy, operate within the chosen channels, rigorously measure the outcomes, and then use those insights to refine and enhance the engagement approach. * **Harmonized Customer Experience:** The overarching objective is to create a consistent, harmonized customer experience across all touchpoints, ensuring that interactions are always relevant and driven by data-backed insights rather than siloed or inconsistent efforts. **Tools/Resources Mentioned:** * **Veeva (Viva):** Explicitly mentioned as the foundational platform for CRM and insights generation within the pharmaceutical company. **Key Concepts:** * **Harmonized Customer Experiences:** The goal of ensuring that all customer interactions, regardless of channel (field rep, digital content, etc.), are consistent, relevant, and aligned with the customer's needs and journey. * **Moments That Matter:** Specific, high-impact points in the customer (HCP or patient) journey where timely and relevant information can significantly influence outcomes or decisions, such as immediately post-diagnosis or during complex clinical inquiry.

Gaining Greater Strategic Value from Events
Veeva Systems Inc
@VeevaSystems
Aug 9, 2017
This video provides an in-depth look into how Teva, a global pharmaceutical company, strategically manages its massive volume of commercial and medical events to maximize go-to-market effectiveness. Events are positioned as a crucial component of Teva’s customer engagement model, particularly for launching new products and establishing market presence. The sheer scale of their operation is significant, with the company executing approximately 8,000 events annually, necessitating a highly streamlined and efficient management process to ensure operational success. The primary challenge Teva faced was achieving harmonization across its diverse, global organizational structure. Operating across different business units—specifically generics, over-the-counter (OTC) products, and specialty/medical products—each unit historically ran its events using disparate approaches and processes. This lack of standardization created complexity and inefficiency. To address this, Teva sought a globally scalable software platform capable of unifying these varied processes under a single, cohesive system. The objective was to make the event management process as easy and streamlined as possible for all stakeholders, from global administrators to field-based sales representatives. Teva ultimately selected Veeva Event Management, citing its suitability as the best-fitting software solution that offered full integration into their existing CRM infrastructure. The selection criteria were multifaceted, balancing user experience with regulatory necessity. A core requirement was empowering sales representatives in the field—who frequently use iPads—with an easy-to-use tool for organizing and executing events. However, the highest consideration, often overlooked until the end of the selection process, was the system’s capability to ensure complete transparency and regulatory compliance. Teva emphasized that while ease-of-use is critical for adoption, the system’s ability to manage compliance effectively was paramount and should be treated as the foundational requirement. This case study highlights the critical intersection of commercial strategy, operational efficiency, and regulatory adherence within the life sciences sector. By integrating event management directly into their CRM, Teva aimed not only to simplify field execution but also to build a robust, auditable framework that supports their high volume of market activities while meeting stringent global compliance standards. The successful harmonization across disparate business units (generics, OTC, specialty) demonstrates a strategic move toward leveraging centralized technology to drive consistent, compliant commercial engagement worldwide. Key Takeaways: • **Strategic Importance of Events:** Events are not merely logistical tasks but are considered crucial go-to-market activities, serving as the best method for presenting new products and driving customer engagement in the pharmaceutical sector. • **Scale Demands Streamlining:** Teva manages approximately 8,000 events per year, illustrating the immense operational scale required in large pharma commercial operations, which necessitates highly automated and streamlined management solutions. • **Harmonization is Key to Global Efficiency:** A major challenge for global organizations like Teva is harmonizing event management processes across different business units (generics, OTC, specialty/medical) that historically operate differently; a centralized platform is essential for consistency. • **Integration with CRM is Mandatory:** The decision to choose Veeva Event Management was heavily influenced by its capability to be fully integrated into Teva's existing CRM system, ensuring data continuity and maximizing the existing technology investment. • **Dual Focus: Usability and Compliance:** System selection must balance two critical requirements: ease of use for field representatives (e.g., mobile usability on the iPad) and robust capabilities for transparency and regulatory compliance. • **Compliance as the Highest Consideration:** While user experience drives adoption, compliance capabilities must be prioritized as the foundational requirement for any regulated enterprise software, ensuring auditability and adherence to global standards. • **Empowering the Field:** The chosen system must provide sales representatives with an easy, intuitive way to manage events directly from the field, minimizing administrative burden and maximizing time spent on customer engagement. • **Centralized Platform for Decentralized Execution:** Utilizing a global software platform allows a large organization to maintain centralized control, data integrity, and compliance standards while supporting decentralized execution across various international business units. Tools/Resources Mentioned: * **Veeva Event Management:** The specific software platform chosen by Teva for harmonizing and managing its global events. * **Existing CRM System:** The platform into which Veeva Event Management was fully integrated (implied to be Veeva CRM, given the channel and context). * **iPad:** Mentioned as the primary device used by sales representatives in the field, highlighting the need for mobile-optimized solutions. Key Concepts: * **Go-to-Market Activities:** Commercial strategies and actions taken to introduce a product or service to a new market, heavily relying on events for initial presentation and engagement. * **Harmonization:** The process of standardizing disparate processes, systems, or approaches across different business units or geographies within a large organization to achieve consistency and efficiency. * **Transparency and Compliance:** The critical regulatory requirement in the pharmaceutical industry to ensure that all commercial activities, particularly events involving healthcare professionals, are fully documented, auditable, and adhere to industry regulations. Examples/Case Studies: * **Teva Case Study:** Teva, a global pharmaceutical company, successfully implemented Veeva Event Management to harmonize the event processes for its three distinct business units: Generics, OTC, and Medicals/Specialty. * **8,000 Events Annually:** Specific data point illustrating the massive scale of Teva’s reliance on events for customer engagement and product promotion.

2016 Veeva Commercial Summit
Veeva Systems Inc
/@VeevaSystems
Jul 28, 2017
This video captures the opening moments and atmosphere of the 2016 Veeva Commercial Summit, an event explicitly positioned as the largest gathering for commercial and medical affairs professionals within the life sciences industry. The primary focus of the brief segment is to establish the scale and importance of the gathering, serving as a high-energy welcome to attendees. The speaker emphasizes the breadth of participation, noting the presence of over 140 companies, encompassing both large pharmaceutical corporations and smaller biotech organizations, all operating within the life sciences ecosystem. The core message conveyed is one of industry vitality and collective action. The speaker directly addresses the audience, identifying them as the key personnel—the "people that are making things happen"—who drive innovation and progress within the sector. This introductory segment sets the stage for a conference dedicated to sharing information, discussing best practices, and collaboratively advancing the commercial and medical strategies crucial for drug development, regulatory adherence, and patient outreach. The use of music and a vibrant welcome aims to foster a sense of community and shared purpose among the attendees who are focused on optimizing commercial operations, a key area for specialized consulting services. While the provided transcript is purely introductory and lacks specific technical or strategic content, its value lies in confirming the foundational importance of the Veeva platform as a central hub for commercial operations in 2016. The event’s focus on commercial and medical affairs aligns precisely with the departments IntuitionLabs.ai targets with its AI-powered sales operations assistants and medical information chatbots. The context confirms the ongoing need for specialized technology and consulting services to manage the complex data and regulatory requirements inherent in these commercial functions, highlighting the enduring relevance of optimizing Veeva systems. Key Takeaways: * **Veeva’s Centrality in Life Sciences Commercial Strategy:** The sheer scale of the 2016 Summit (over 140 companies represented) underscores Veeva’s established position as the dominant enterprise software provider for commercial and medical affairs, reinforcing the strategic necessity of offering specialized Veeva CRM consulting services. * **Confirmation of Target Audience Focus:** The event’s explicit focus on commercial and medical affairs professionals confirms these groups as the primary decision-makers and end-users for AI-driven solutions, such as Generative AI Sales Ops Assistants and Medical Info Chatbots. * **Historical Context for Digital Transformation:** Analyzing content from the 2016 summit provides valuable historical context regarding the state of commercial technology adoption before the widespread integration of advanced LLMs, helping to benchmark the current pace of digital transformation in the sector. * **Industry Collaboration and Information Sharing:** The stated goal of the summit—to "share a lot of information and move the industry forward"—highlights the continuous need for innovative solutions and knowledge transfer, presenting opportunities for IntuitionLabs.ai to position itself as a thought leader in AI implementation within this ecosystem. * **Focus on Operational Execution:** The emphasis on attendees as the "people that are making things happen" reinforces that the core challenge addressed by Veeva (and by IntuitionLabs.ai’s services) is the optimization and execution of commercial operations, data management, and field force effectiveness. * **Market Penetration Across Company Sizes:** The attendance includes "everyone large and small in the life sciences industry," confirming that the need for robust CRM solutions and subsequent AI optimization extends beyond top-tier pharmaceutical companies to include smaller biotech and emerging life sciences firms. * **Enduring Relevance of Commercial and Medical Affairs Integration:** The joint focus of the summit on both commercial and medical affairs indicates the critical need for integrated systems and data flows between these two regulated departments, a challenge AI and data engineering services are uniquely positioned to solve. * **Opportunity for AI-Driven Modernization:** While the 2016 context predates current LLM capabilities, the foundational challenges discussed at the summit—data management, field force effectiveness, and compliance—represent ripe opportunities for IntuitionLabs.ai to introduce modern AI agents and automation tools.

Veeva Network's Profiles Help You Understand Healthcare Professionals
Veeva Systems Inc
/@VeevaSystems
Jul 20, 2017
This video provides an in-depth exploration of Veeva Network’s Healthcare Professional (HCP) profiles, positioning Network as a foundational master data management (MDM) solution essential for compliant, multichannel customer engagement in the life sciences industry. The presentation establishes that achieving a complete, accurate customer view is crucial but challenging, and Veeva Network simplifies this process by managing and delivering accurate, up-to-date HCP, Healthcare Organization (HCO) profiles, and affiliation data. A core theme is Network’s unique architecture, which includes a single global data model, seamless integration capabilities with both upstream and downstream data sources, and deep integration with Veeva CRM as part of the broader Veeva Commercial Cloud. The presentation details several differentiating capabilities of Veeva Network beyond traditional enterprise customer MDM solutions. These include a powerful relationship discovery tool that allows for visual exploration of complex HCP and HCO affiliations and hierarchies, which is critical for understanding influence networks. Furthermore, the system is designed for global control with local flexibility, featuring built-in, configurable multi-country management to handle diverse regulatory and market requirements worldwide. The demonstration focuses on the structure of an individual HCP profile, illustrating how the system combines proprietary data (Veeva Open Data) with client-specific custom fields, managed through distinct data stewardship processes. Using the example of Dr. Javier Sanchez, a practicing physician, the video highlights the comprehensive nature of the profile data. The profile aggregates essential demographics (e.g., prescriber status, degrees, primary and secondary specialties) sourced from Veeva Open Data. Crucially, the profile manages multiple address types, including professional addresses based on admitting rights, preferred mailing addresses, and historical information, ensuring engagement is directed accurately. The system tracks current, inactive, and historical parent/primary affiliations, allowing commercial teams to understand the full scope of an HCP’s institutional relationships. Finally, the profile includes critical compliance and engagement data, such as a verified email address for approved digital outreach, pre-integrated industry identifiers for cross-referencing with external data sources, and federal and state licensing information, which is explicitly noted as enabling rapid sample eligibility determination. The overall approach emphasizes how robust, well-governed master data is the prerequisite for effective commercial operations and regulatory adherence. By clearly delineating between globally managed data (orange fields, handled by Veeva data stewardship) and locally managed custom data (gray fields, handled by local stewards), Veeva Network provides a clear governance framework. This structure ensures data quality and consistency while allowing pharmaceutical companies the necessary flexibility to incorporate their unique internal data assets, ultimately maximizing the effectiveness of their Veeva CRM investment and multichannel engagement strategies. Key Takeaways: • **Foundational MDM for Commercial Success:** Veeva Network serves as the central master data management solution for the life sciences industry, providing accurate HCP and HCO profiles necessary for compliant and effective multichannel customer engagement. • **Seamless Veeva Integration:** Network is deeply integrated with Veeva CRM as part of the Veeva Commercial Cloud, ensuring that sales and marketing teams always operate using the most current, verified customer data directly within their workflow tools. • **Dual Data Governance Model:** HCP profiles utilize a clear distinction between "orange fields" (default data provided by Veeva Open Data, managed by Veeva stewardship) and "gray fields" (custom, client-specific data, managed by local data stewards), ensuring both global consistency and local relevance. • **Compliance-Critical Data Points:** The profiles house essential regulatory data, including federal and state licensing information, which is explicitly used to facilitate rapid and accurate sample eligibility determinations, a key regulatory requirement for pharmaceutical commercial operations. • **Relationship Discovery and Hierarchy Mapping:** Veeva Network includes a powerful tool for visually exploring complex affiliations and hierarchies between HCPs and HCOs, allowing commercial and medical affairs teams to map influence networks and organizational structures. • **Comprehensive Address Management:** The system manages multiple address types (e.g., professional addresses based on admitting rights, preferred mailing addresses, historical addresses) to ensure accurate physical engagement and mailing compliance. • **Global Control with Local Flexibility:** The platform supports multi-country management, allowing global pharmaceutical companies to maintain a standardized data model while configuring local variations necessary for different market regulations and data sources. • **Enabling Approved Digital Engagement:** Profiles track verified email addresses and other contact information, ensuring that digital engagement channels (like approved email) are utilized based on accurate and verified contact data. • **Industry Identifier Integration:** The profiles incorporate pre-integrated industry identifiers, simplifying the process of cross-referencing and integrating data from various external industry data sources with the internal master record. • **Historical Data Retention:** Veeva Network maintains historical and inactive data (e.g., past addresses, former affiliations), providing a complete audit trail and comprehensive view of the HCP’s professional history, which is valuable for compliance and longitudinal analysis. Tools/Resources Mentioned: * Veeva Network * Veeva CRM * Veeva Commercial Cloud * Veeva Open Data Key Concepts: * **HCP Profile:** A comprehensive, single source of truth record for a Healthcare Professional, aggregating demographic, professional, affiliation, and licensing data. * **HCO Profile:** A corresponding record for a Healthcare Organization (e.g., hospital, clinic). * **Master Data Management (MDM):** The discipline of managing and ensuring the accuracy, consistency, and completeness of critical data assets (like customer profiles) across an enterprise. * **Veeva Open Data:** The proprietary, verified data source provided by Veeva that populates the default fields (orange fields) in Network profiles. * **Data Stewardship:** The process of managing and ensuring the quality and integrity of data, handled either by Veeva (for Open Data fields) or by the client (for custom fields). * **Sample Eligibility Determination:** The process of verifying an HCP’s licensing status and credentials to ensure they are legally eligible to receive pharmaceutical samples, a critical regulatory compliance step. Examples/Case Studies: * **Dr. Javier Sanchez Profile:** Used as a practical example of an HCP profile in the Albany, New York area. The profile demonstrated the aggregation of data points such as: * Prescriber status and Doctor of Medicine degree. * Primary specialty (Pediatrics) and other specialties. * Multiple address types (professional based on admitting rights, preferred mail). * Current affiliations (Albany Medical Center Hospital and College). * Verified email address for approved engagement. * Federal and state licensing information.

Enhance Visibility and Manage Content Across Your Digital Supply Chain
Veeva Systems Inc
@VeevaSystems
Jul 20, 2017
This video, presented by Veeva Systems, outlines the challenges faced by pharmaceutical commercial and medical affairs teams in managing and distributing content across a complex digital supply chain and demonstrates how Veeva Vault PromoMats solves these issues. The core problem illustrated is the fragmentation of content, which resides in multiple systems, leading to slow approval cycles, unnecessary duplication of effort across global regions, and difficulty ensuring that only compliant, approved materials reach the customer through the correct channels. The scenario follows Jessica, who is launching a new product (Vecchio) and needs to create, approve, and distribute various types of content efficiently while coordinating with external agencies and internal global colleagues. The solution presented centers on integrating the content lifecycle using Veeva Vault PromoMats. By leveraging this unified platform, Jessica can upload all digital assets and associated metadata, streamlining the process from agency briefing to final distribution. The system facilitates rapid selection of the internal approval team and, crucially, automatically applies appropriate meta tags and usage rights upon approval. This integration is vital for global efficiency, as colleagues like James and Rosa in Europe and Latin America can instantly access and appropriately reuse approved content, eliminating the need to recreate similar assets from scratch. This global collaboration capability significantly accelerates time-to-market for regional teams. Furthermore, the platform ensures rigorous regulatory adherence and provides critical business intelligence. Once approved, the content is distributed to field teams with a single click, all while maintaining a comprehensive, immutable audit trail—a necessity for GxP and FDA compliance. The system also provides visibility into content performance, allowing Jessica and her colleagues to track where and how their assets are being used and measure their impact on the business. Finally, when assets reach the end of their life cycle, they are automatically archived in the cloud, ready to be accessed for compliance audits, ensuring that only the latest, compliant materials are ever available for distribution. This comprehensive approach transforms content management from a fragmented compliance risk into an efficient, transparent, and globally scalable operation. Key Takeaways: • **Fragmentation Hinders Commercial Speed:** Traditional content management systems often house assets in multiple disparate locations, severely slowing down the ability of commercial teams (like Jessica) to create, approve, and distribute materials necessary for new product launches. • **Veeva Vault PromoMats as the Integrated Hub:** The platform serves as a single, centralized repository for all digital assets, enabling seamless workflow management from agency creation through internal approval and final distribution, thereby speeding up the entire content supply chain. • **Global Content Reuse is Critical for Efficiency:** The system facilitates global collaboration by allowing regional teams (like those in Europe and Latin America) to instantly access and reuse approved content from other regions, eliminating redundant asset creation and ensuring consistency across markets. • **Mandatory Audit Trail for Compliance:** A core feature is the automatic maintenance of a full audit trail throughout the content lifecycle, which is essential for meeting stringent regulatory requirements (e.g., FDA, GxP) and demonstrating compliance during audits. • **Metadata and Usage Rights Automation:** Approved content is automatically stored with appropriate meta tags and usage rights flagged, ensuring that assets are used correctly and only in channels for which they have been approved. • **Enhanced Visibility and Business Insights:** The platform provides commercial teams with actionable insights by tracking where and how their approved assets are being utilized in the market, allowing them to measure the business impact of their content strategy. • **Ensuring Distribution Compliance:** PromoMats guarantees that only the latest, globally approved version of content is available for distribution to field teams and customers, mitigating the risk of non-compliant or outdated materials reaching the market. • **Streamlined Distribution to Preferred Channels:** The system enables quick distribution to field teams and ensures that customers can view the content through their preferred digital channels, optimizing the customer experience and engagement. • **Automated Content Archiving:** Assets are automatically archived in the cloud upon reaching the end of their life cycle, ensuring that they are preserved and readily accessible for future compliance checks and regulatory inquiries. • **Transparency and Stakeholder Collaboration:** The integrated platform enhances transparency, allowing the global team to work efficiently with all stakeholders—internal approvers, external agencies, and regional colleagues—on a single source of truth. Tools/Resources Mentioned: * Veeva Vault PromoMats * Salesforce (mentioned as the traditional system being replaced/integrated) Key Concepts: * **Digital Supply Chain:** Refers to the end-to-end process of creating, approving, distributing, and managing digital content (rich media, materials) from the initial concept through to the customer and final archiving. * **Audit Trail:** A chronological record of system activities, including who accessed or modified content and when, which is mandatory for demonstrating regulatory compliance in the life sciences industry. * **Meta Tags and Usage Rights:** Descriptive data (metadata) applied to digital assets that defines their content, context, and, crucially, the specific channels, regions, and timeframes for which they are legally and commercially approved.

PromoMats Video: Zinc to PromoMats Migration
Veeva Systems Inc
/@VeevaSystems
Jul 20, 2017
This video outlines the streamlined and compliant methodology for migrating pharmaceutical content management systems from Zinc to Veeva Vault PromoMats. The core purpose of the presentation is to assure users that while moving enterprise systems can be daunting, the transition to Vault PromoMats has been engineered to be simple, compliant, and rapid, allowing life sciences organizations to quickly leverage the advanced functionalities of the industry-leading platform. The speaker emphasizes that PromoMats is the central hub for commercial content management, offering significant advantages over legacy systems, including three major system upgrades per year, enhanced workflow capabilities, more advanced reporting tools, and robust Digital Asset Management (DAM) features. The migration process itself is defined by a clear, three-step methodology designed to minimize disruption and ensure regulatory adherence. Step one involves the direct transfer of essential foundational data: the reference library, user profiles, product definitions, and job fields are moved into a new Vault PromoMats instance. This ensures that the structural integrity required for compliant content review and approval (MLR) is maintained from the outset. Step two focuses on configuration and validation, where a team of experts completes and validates the specific system configuration tailored to the client’s needs. This validation step is crucial in a regulated environment, confirming that the new system meets GxP and internal compliance standards before go-live. The final step addresses user readiness through a comprehensive training package. This includes webinar training, pre-recorded Vault PromoMats demonstrations, access to online help resources, and a range of supporting training materials, ensuring rapid user adoption and proficiency. Crucially, the video addresses the challenge of managing legacy content for compliance purposes. While day-to-day activity immediately shifts to the new Vault PromoMats, all materials previously created in Zinc are maintained in a secure Zinc archive site. This archive remains accessible for business-critical activities, such as managing reapprovals or conducting audits of legacy materials, until a specified cutoff date (mentioned as the end of 2020 in the transcript). After this period, the complete historical data is provided to the client on an encrypted hard drive, ensuring perpetual access for long-term regulatory compliance requirements, allowing organizations to fully embrace the user-focused, fast, and easy features of Veeva PromoMats. Key Takeaways: * **Compliant Migration Path:** The transition from Zinc to Veeva Vault PromoMats is structured as a simple, three-step process specifically designed to maintain regulatory compliance throughout the migration, which is critical for pharmaceutical commercial operations. * **Enhanced Platform Capabilities:** Vault PromoMats offers substantial functional improvements over Zinc, including three major system upgrades annually, enhanced workflow automation, sophisticated reporting capabilities, and integrated Digital Asset Management (DAM). * **Defined Data Transfer Scope:** The initial migration focuses on moving core foundational elements necessary for compliant operations: the reference library, user accounts, product definitions, and job fields are ported directly into the new Vault instance. * **Expert Configuration and Validation:** A dedicated team of experts is responsible for completing and validating the specific Vault configuration, ensuring that the system is properly set up and meets all necessary regulatory and internal quality standards before deployment. * **Comprehensive Training Strategy:** User adoption is supported by a multi-faceted training package, including live webinar sessions, pre-recorded demonstrations, online help resources, and various training materials to accelerate user proficiency. * **Legacy Content Archival for Audits:** All materials created in the legacy Zinc system are securely archived, remaining accessible for business-critical activities such as managing reapprovals and conducting regulatory audits of historical content. * **Perpetual Data Access:** Post-archival period, the complete historical data set from Zinc is provided to the client on an encrypted hard drive, ensuring the organization retains perpetual access to legacy materials for long-term compliance and audit trail requirements. * **Focus on Commercial Content Management:** The migration targets the core of commercial content management, enabling faster, more efficient, and more compliant processes for marketing and promotional materials review and approval (MLR). Tools/Resources Mentioned: * **Veeva Vault PromoMats:** The target platform for commercial content management, offering advanced workflows and digital asset management. * **Zinc:** The legacy content management system being migrated away from. * **Secure Zinc Archive Site:** A dedicated site for retaining legacy content for audit and reapproval purposes during the transition period. * **Encrypted Hard Drive:** The medium used to transfer the complete historical Zinc data set to the client for permanent retention after the archive period concludes. Key Concepts: * **Digital Asset Management (DAM):** Integrated capabilities within Vault PromoMats for managing and organizing digital content assets. * **Configuration Validation:** The crucial process of verifying that the new system setup adheres to specified requirements and regulatory standards (essential for GxP environments). * **Reapprovals and Audits:** Business-critical activities requiring access to legacy content, which are supported by the secure Zinc archive during the transition phase. * **Reference Library:** A core data structure within content management systems containing approved claims, sources, and supporting documentation necessary for compliant content creation.

Veeva OpenData Email: Extend Your Reach
Veeva Systems Inc
/@VeevaSystems
Jul 20, 2017
The video details the strategic advantages of utilizing Veeva OpenData Email for pharmaceutical and life sciences companies seeking to optimize their multi-channel marketing campaigns and improve engagement with healthcare professionals (HCPs). The presentation frames the challenge through the experience of Vivianne, a brand marketing manager at Ver Teo Biopharma, who requires a consistent customer experience across all interactions while needing to reach practitioners inaccessible to her sales representatives. Historically, Vivianne relied on multiple third-party email lists, which resulted in fragmented pricing, restrictive usage agreements, and poor campaign performance characterized by high bounce rates and low open/click-through rates. Veeva OpenData is presented as the definitive solution to these common commercial operations hurdles. The platform provides access to millions of verified HCP email addresses, encompassing all prescriber types and specialties. A core element of its value proposition is the superior quality of the data, which is sourced from trusted industry organizations and actively stewarded by Veeva. This rigorous data management process ensures consistent quality and reliability, demonstrated by a reported email deliverability rate of nearly 100% in a recent test. This high deliverability directly addresses the performance issues associated with conventional email lists, allowing marketers like Vivianne to achieve acceptable campaign results. Beyond data quality, the video highlights the commercial and administrative benefits of switching to Veeva OpenData. The solution offers predictable email pricing, granting Vivianne greater control over her monthly spending. Furthermore, the platform simplifies legal and procurement complexity by providing unlimited usage rights for a full year under a single agreement, eliminating the restrictions inherent in managing multiple vendor contracts. Ultimately, adopting Veeva OpenData allows email to function as a reliable, foundational element of the marketing mix, ensuring that customers receive consistent and useful information from the biopharma company, regardless of the touchpoint (office or web), thereby strengthening the overall multi-channel strategy. Key Takeaways: • **Enhanced HCP Reach:** Email remains a critical component of the multi-channel strategy, particularly for reaching healthcare practitioners (HCPs) who are not regularly contacted by the field sales force, thus extending the company's overall market reach. • **Mitigating Third-Party Data Risks:** Pharmaceutical marketing teams often struggle with high bounce rates and poor performance metrics (low open/click-through rates) due to reliance on fragmented, low-quality email lists purchased from multiple vendors. • **Data Quality Assurance:** Veeva OpenData ensures superior data quality by sourcing email addresses from trusted industry organizations and actively stewarding the data records, which is crucial for maintaining compliance and campaign effectiveness. • **Near-Perfect Deliverability:** The commitment to data quality translates into tangible performance benefits, with the video citing a recent test confirming email deliverability of nearly 100%, significantly boosting campaign efficiency. • **Comprehensive HCP Coverage:** The platform offers access to millions of verified HCP email addresses, covering the full spectrum of prescriber types and specialties, providing a robust foundation for highly targeted marketing efforts. • **Budget and Pricing Predictability:** Veeva OpenData simplifies financial planning for commercial operations by offering predictable email pricing, granting marketing managers better control over monthly spending compared to variable vendor costs. • **Simplified Usage Rights:** The solution eliminates the complexity of managing multiple data usage restrictions by providing unlimited usage of the verified email data for a full year under a single, straightforward agreement. • **Foundational Marketing Element:** High-quality, reliable email data allows marketers to confidently integrate email as a foundational element of their marketing mix, supporting the delivery of consistent and useful information to customers across digital and physical channels. • **Optimization of Commercial Operations:** By improving data quality, simplifying procurement, and boosting deliverability, Veeva OpenData directly contributes to the optimization of pharmaceutical commercial operations, aligning with goals for maximizing CRM and marketing technology investments. Tools/Resources Mentioned: * **Veeva OpenData Email:** The specific product discussed, providing verified HCP email addresses for multi-channel marketing campaigns. Key Concepts: * **Multi-Channel Strategy:** The integrated approach to customer engagement that ensures a unified and consistent experience across all communication vectors (e.g., sales reps, email, web). * **Data Stewardship:** The active and professional management of data records, including verification and maintenance, to ensure their accuracy and reliability over time, which is the mechanism Veeva uses to guarantee high deliverability. * **Email Deliverability:** A key performance indicator (KPI) measuring the success rate of emails reaching the intended recipient's inbox, which is directly impacted by the quality and verification status of the email list.

Veeva Vault QualityDocs
Veeva Systems Inc
/@VeevaSystems
Jul 13, 2017
This video explores Veeva Vault QualityDocs, a regulated content management application specifically designed for the life sciences industry. The speaker demonstrates how the platform manages the entire GxP document lifecycle, from initial drafting and multi-stage approvals (including QA sign-off) to training, periodic review, controlled copy distribution, and compliant change control. The presentation highlights the system's focus on regulatory compliance, ease of use, and operational efficiency for pharmaceutical and biotech companies. Key Takeaways: * **Comprehensive GxP Document Lifecycle Management:** Veeva Vault QualityDocs provides an end-to-end solution for GxP document control, encompassing creation, multi-stage review and approval, automated training assignments, periodic review workflows, and compliant change control processes, ensuring regulatory adherence throughout. * **Robust Regulatory Compliance Features:** The platform natively supports critical compliance requirements, including 21 CFR Part 11 compliant electronic signatures with detailed audit trails, controlled copy functionality for secure external distribution with unique tracking, and configurable document watermarks and headers/footers. * **Streamlined Workflows and Automation:** Vault QualityDocs leverages pre-designed configurations (document types, workflows, lifecycles) tailored for life sciences quality processes, enabling automated tasks such as training assignments, periodic review reminders, and gatekeeper-style change control initiation, reducing manual effort and ensuring process consistency. * **Enhanced Accessibility and User Experience:** Designed as a zero-footprint, browser-agnostic application with a consumer web-inspired interface, Vault QualityDocs prioritizes ease of use and accessibility from any device, facilitating user adoption and efficient process execution without extensive training. * **Integrated Reporting and Auditability:** The system offers built-in, point-and-click reporting capabilities for tracking compliance (e.g., training completion, controlled copy distribution) and maintains a comprehensive audit trail that records all document activities, property changes, and electronic signatures, which is crucial for regulatory inspections. * **Multi-Tenant Cloud for Operational Relief:** Delivered via a multi-tenant cloud, Veeva handles all infrastructure, upgrades, backups, and disaster recovery, relieving clients from operational IT concerns and ensuring that critical regulatory updates (e.g., to 21 CFR Part 11) are prioritized and integrated into the platform.

Extending Quality Processes to Your Global Workforce
Veeva Systems Inc
/@VeevaSystems
Jul 13, 2017
This video provides an in-depth exploration of extending quality processes to a global workforce using Veeva Vault QualityDocs. Jeff Shiran, a Sales Engineer for R&D at Veeva, demonstrates how the Vault platform facilitates compliant and collaborative document management within the pharmaceutical and life sciences industries. The presentation highlights the unique advantages of Vault's cloud-based architecture, emphasizing its built-in compliance features and user-friendly design, which are crucial for managing GxP documents and ensuring regulatory adherence across diverse teams and external partners. The core of the discussion revolves around three main pillars: accessibility, consumer web design, and a multi-tenant cloud solution. Vault's web-browser accessibility allows users to interact with the system from any device, promoting flexible work arrangements. The platform's design draws inspiration from consumer web experiences, making it intuitive to find and manage documents, akin to online shopping platforms. Furthermore, as a multi-tenant cloud solution, Veeva Vault ensures all clients are on the same version, enabling rapid innovation and significantly reducing the validation burden traditionally associated with regulated enterprise software. A significant portion of the demonstration focuses on secure collaboration with both internal and external stakeholders. Shiran illustrates how Vault can manage granular permissions for different user types, from internal quality directors and associates to external consultants and contract manufacturing organizations (CMOs). The system allows for controlled sharing of documents, ensuring that external parties only access content explicitly permitted, and crucially, all interactions, even with non-Vault users via shared links, are meticulously tracked through a robust audit trail. This capability addresses critical security and compliance concerns, preventing documents from leaving the controlled environment without traceability. The demo walks through practical scenarios, including creating and authoring GxP documents, managing review and approval workflows, and utilizing dashboards for real-time insights into document status and overdue tasks. Shiran showcases how document properties can automate workflows and how the system auto-generates viewable PDF renditions, eliminating the need for native software. The session concludes by addressing security concerns, emphasizing how Veeva's cloud infrastructure and inherent auditability enhance compliance and reduce the risk of uncontrolled document sharing compared to older, VPN-dependent systems. Key Takeaways: * **Veeva Vault's Inherent Compliance:** The Vault platform is designed from the ground up for life sciences, incorporating features like 21 CFR Part 11 compliant signatures, PDF publishing, and robust audit trails, eliminating the need for retrofitting generic systems. * **Global Accessibility and Device Independence:** Users can access Veeva Vault QualityDocs from any device (PC, Mac, mobile) with just an internet connection and a web browser, facilitating collaboration across a geographically dispersed workforce. * **Intuitive User Experience:** Leveraging "consumer web design" principles, Vault offers an easy-to-use interface for finding and managing documents, improving user adoption and reducing the learning curve. * **Multi-Tenant Cloud Benefits:** Being a multi-tenant cloud solution allows Veeva to rapidly innovate and deploy new features, while also significantly lessening the validation burden for client companies. * **Granular Permission Management:** Vault enables precise control over document access and functionality for various user roles, including internal employees (e.g., Director of Quality, Quality Associate) and external partners (e.g., consultants, CMOs). * **Secure External Collaboration:** The system facilitates collaboration with third parties by allowing controlled sharing of specific documents, ensuring external users only see what is intended and for a defined period. * **Robust Audit Trail for All Interactions:** Every action within Vault, including viewing and downloading documents by external, non-Vault users via shared links, is meticulously recorded in a date and time-stamped audit trail, enhancing traceability and compliance. * **Automated Document Workflows:** Document properties (e.g., site, department) can be leveraged to automatically trigger specific review and approval workflows, streamlining quality processes. * **Inline Document Viewing and Rendition Generation:** Users can view documents in an inline browser without needing native software (e.g., Microsoft Word), and Vault automatically generates viewable PDF renditions of source documents. * **Flexible Document Creation:** Users can create new documents from pre-defined templates or upload existing documents from their local systems, with options to classify content later if initial information is incomplete. * **Actionable Business Intelligence Dashboards:** Configurable dashboards provide real-time insights into document status (e.g., by facility) and overdue tasks, allowing for proactive management and global/local oversight. * **Enhanced Security and Auditability:** The cloud-based system offers superior security and auditability compared to legacy systems, preventing uncontrolled document sharing (e.g., via thumb drives) and improving overall compliance. * **Improved User Acceptance for Compliance:** An easy-to-use system like Vault tends to have higher user acceptance, which in turn leads to better compliance as users are less likely to circumvent the system. Tools/Resources Mentioned: * Veeva Vault QualityDocs * Veeva Vault Platform * Microsoft Word (for document authoring) * Google Chrome (web browser used in demo) Key Concepts: * **GxP (Good Practice):** A set of regulations and guidelines for manufacturing, testing, and distributing pharmaceutical products, ensuring quality and safety. * **21 CFR Part 11:** Regulations from the FDA that set forth the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records and handwritten signatures. * **Multi-tenant Cloud Solution:** A single instance of a software application that serves multiple customers (tenants), where data is segregated but all tenants share the same infrastructure and application version. This enables faster updates and shared security investments. * **Audit Trail:** A chronological record of system activities, including who accessed what, when, and what changes were made, essential for regulatory compliance and accountability. * **Viewable Rendition:** An automatically generated, non-editable version (typically PDF) of a source document, used for viewing and sharing without altering the original. * **Inline Browser:** A feature that allows users to view documents directly within the web application interface without downloading them or opening them in external software. * **Consumer Web Design:** Applying principles of user-friendly, intuitive design common in consumer-facing websites to enterprise software to enhance usability and adoption. Examples/Case Studies: * **User Role Demonstrations:** The demo featured four distinct user roles to illustrate permission control: * **Ken Quality (Director of Quality):** A Vault user with high-level access, able to see all documents, create, upload, and manage reports/dashboards. * **Rich Reviewer (Quality Associate):** An internal Vault user with limited access, able to see documents only for his specific site, and perform review/annotation tasks. * **Bob External (External User/Consultant):** An external Vault user with very limited access, only able to see specific assigned documents and perform review/annotation, with no access to reports, dashboards, or creation/upload functions. * **Jeff CMO (Contract Manufacturing Associate):** A non-Vault user who receives a time-limited, trackable link to view a document, with all his interactions (viewed, downloaded) recorded in Vault's audit trail. * **Document Workflow:** Illustrated the process of creating an SOP from a template, checking it out for authoring in Microsoft Word, checking it back in, and initiating a review and approval cycle involving different internal and external users. * **Dashboard Reporting:** Showcased a "Global Dashboard" for document status across the enterprise, with the ability to filter by specific sites (e.g., Chicago) and identify overdue tasks and the responsible individuals. * **Security Feedback:** A client anecdote highlighted how Vault's audit trail and controlled access prevented the common issue of employees putting sensitive documents on thumb drives or emailing them outside the system for home access, thereby enhancing security and compliance.

Simplifying Role-based Readiness
Veeva Systems Inc
/@VeevaSystems
Jul 13, 2017
This video explores how Veeva Vault QualityDocs simplifies role-based readiness and training for regulated documents within the life sciences industry. The speaker demonstrates how users can easily complete "read and understood" training tasks via email or a personalized home screen, ensuring 21 CFR Part 11 compliant electronic signatures. Managers can leverage built-in, point-and-click reporting and dashboarding tools to gain a 50,000-foot view of training compliance across users, job roles, and specific documents. The discussion also highlights Veeva Vault's capabilities as a digital asset library, supporting various content types including video for training, and its open API for integration with external Learning Management Systems (LMS) to facilitate more advanced, Level 2 training. The overarching theme is the platform's ability to combine ease-of-use with robust compliance for quality processes in regulated environments. Key Takeaways: * **Regulated Content Management Foundation:** Veeva Vault is presented as a purpose-built platform for the life sciences, offering inherent productivity and compliance features (e.g., 21 CFR Part 11 signatures, audit trails) for managing regulated content, including GxP documents. * **Streamlined "Read and Understood" Workflows:** The system automates the assignment, notification, and completion of training tasks, allowing users to easily access and acknowledge documents, thereby enhancing training compliance and operational efficiency. * **Powerful Compliance Reporting & Dashboards:** Veeva Vault provides intuitive, configurable reporting and dashboarding tools that enable managers to quickly track training compliance by individual, job role, or document, offering immediate insights into readiness and potential gaps. * **LMS Integration Capabilities:** The platform supports integration with external Learning Management Systems (LMS) via an open API, allowing for the extension of basic "read and understood" training to more complex Level 2 training (e.g., quizzes, curriculums) while maintaining a comprehensive audit trail. * **Versatile Digital Asset Support:** Veeva Vault functions as a digital asset library, capable of handling and rendering diverse training materials, including video, directly within the browser, offering flexibility in content delivery. * **Reduced Validation Burden:** As a multi-tenant cloud-based solution, Veeva Vault minimizes the implementation and upgrade burden, particularly by assisting with validation efforts (e.g., OQ), which is a significant advantage for regulated pharmaceutical and life sciences companies.

Extending Quality Process Across Organization
Veeva Systems Inc
/@VeevaSystems
Jul 13, 2017
The webinar, hosted by Veeva Systems, provides an in-depth look at the advanced capabilities of **Veeva Vault QualityDocs**, focusing on how the platform extends quality processes beyond basic document management to encompass enterprise-wide collaboration and diverse digital asset control. The presentation establishes Vault as a compliance-ready, productivity-focused system specifically engineered for the life sciences industry, contrasting it with previous generation systems that often required extensive retrofitting to meet regulatory standards like 21 CFR Part 11. A significant portion of the demonstration focuses on the platform's architecture, highlighting that Vault is built on a multi-tenant cloud model. This approach is presented as a major advantage, as Veeva handles the heavy lifting of validation and upgrades, allowing clients to focus on their core operations while maintaining adherence to GxP and other regulatory requirements. The system is designed for accessibility, requiring only an internet connection and a browser, making it device and operating system agnostic. The user experience is modeled after the "consumer web" (e.g., Amazon.com), utilizing powerful text search combined with faceted filtering based on document metadata (like status, facility, or product) to ensure rapid content retrieval. The core technical feature explored is the use of **Binders**. These are defined as virtual folders that create a logical structure or table of contents for related documents. Crucially, binders only reference the original documents, maintaining a single source of truth across the organization—if a document is updated, the change is reflected everywhere it is referenced. The speaker illustrates how binder templates can be leveraged to automate the creation of complex document packages, such as those required for Computer System Validation (CSV) projects or ISO audits, by automatically pre-populating sections and dropping in necessary document templates (placeholders). Furthermore, the presentation showcases Vault's versatility in managing non-traditional GxP content, including quality label artwork and training videos. For video management, the system allows for inline viewing and real-time collaboration through time-stamped annotations that hyperlink directly to specific moments in the recording, significantly streamlining the review and approval cycle for these assets. The Q&A session confirms the platform’s flexibility, noting that the system supports an unlimited number of configurable document types and properties, enabling highly granular, independent workflows tailored to specific organizational needs, countries, or processes. Key Takeaways: * **Out-of-the-Box Regulatory Compliance:** Veeva Vault QualityDocs is purpose-built for life sciences, incorporating essential compliance features like 21 CFR Part 11 electronic signatures (requiring username, password, and reason), automatic audit trails, and mandatory PDF rendering directly into the platform. * **Reduced Validation Overhead via Cloud Model:** Utilizing a multi-tenant cloud architecture, Veeva manages core system validation and upgrades, significantly decreasing the validation burden and IT maintenance costs for pharmaceutical and biotech clients. * **Binders Ensure Single Source of Truth:** The Binder feature allows users to create logical, structured document packages (e.g., for audits or validation) using references to existing documents, eliminating version control issues and ensuring that all users access the latest approved content. * **Automated Project Setup with Templates:** Binder templates can be used to instantly generate standardized document packages (like CSV binders), automatically creating required sections and inserting placeholder templates for documents such as the Master Validation Plan, accelerating project initiation. * **Unlimited Workflow Customization:** The system supports an unlimited number of configurable document types and subtypes. Each combination can trigger a unique, independent life cycle and workflow, allowing companies to apply highly granular process controls based on content type, facility, or country (e.g., US process vs. Japan process). * **Management of Diverse GxP Assets:** Vault acts as a digital asset library capable of managing non-standard regulated content, including quality label artwork, packaging materials, and training videos, subjecting them to the same robust review and approval processes as SOPs. * **Advanced Video Collaboration:** For training and procedural videos, Vault enables real-time, collaborative review using time-stamped annotations. These comments hyperlink directly to the exact moment in the video, facilitating precise feedback and speeding up the approval cycle for multimedia assets. * **Intuitive Search and Filtering:** The system employs a consumer web-inspired search interface, combining full-text search capabilities with faceted filtering based on document metadata (e.g., status, document type, owning facility) to quickly locate specific content among large volumes of data. * **Support for Nested Hierarchies:** Binders can be nested within other binders to create multi-level document structures (often two to three levels deep), accommodating the organizational complexity of large-scale regulatory submissions or validation projects. Tools/Resources Mentioned: * Veeva Vault Platform * Veeva Vault QualityDocs Key Concepts: * **21 CFR Part 11:** FDA regulation governing the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records and handwritten signatures. * **GxP:** A general term for Good Practices (e.g., Manufacturing, Clinical, Laboratory) that define quality system regulations for life sciences companies. * **Binders:** A virtual content structure within Veeva Vault used to group related documents into a logical package or table of contents, primarily for validation, audit, or submission purposes. * **Multi-Tenant Cloud:** A cloud deployment model where a single software instance serves multiple customers, centralizing maintenance and validation efforts. * **Faceted Filtering:** A search technique allowing users to refine results by selecting criteria based on document metadata (properties).