Videos
Browse videos by topic
All Videos
Showing 1249-1272 of 1435 videos

28b Veeva Insights - Episode 3: Functional content for better sales outcomes
twentyeightb
/@twentyeightb
May 13, 2019
This video provides an in-depth exploration of leveraging functional content within Veeva CLM (Content Lifecycle Management) for enhanced sales outcomes in the pharmaceutical industry. The speaker, James Harper, founder of twentyeightb, aims to help brand teams and medical communications agencies better understand and utilize the Veeva CRM platform. The episode focuses specifically on "functional interactive content" for Veeva CLM multi-channel presentations, often referred to as eDetailers, eSales Aids, or digital sales aids. The core of the discussion revolves around defining and demonstrating the power of "functional content" compared to static or basic interactive content. Functional content is described as content that "works for a living," actively engaging both the user (Key Account Manager) and the customer (healthcare professional). It calculates, measures, records, and personalizes information to maximize impact, going beyond simple navigation or pop-ups. This advanced content not only drives greater customer engagement but also provides valuable market insights, monitors content effectiveness, and significantly enhances sales effectiveness. A practical example is demonstrated using a hypothetical product that has secured a new pediatric indication. The critical success factor for this brand is to increase the recognition and treatment of children with this condition. The functional content enables a Key Account Manager (KAM) to initiate a sales call by asking a General Practitioner (GP) to prioritize various patient types (e.g., elderly, pregnant mothers, pediatrics) they see with the condition. This interactive prioritization, where the GP can drag and drop patient groups into an order reflecting their current practice, allows the KAM to understand the GP's perspective on treating children. This data is then saved against the customer's record within the Veeva CRM. This saved, personalized data is crucial as it can dynamically drive the rest of the presentation, ensuring the content is tailored to the GP's specific priorities. The same personalized priority order can reappear at the end of the presentation, enabling the KAM to close with a targeted commitment from the doctor, such as reprioritizing pediatric treatment based on the evidence presented. Furthermore, this data powers subsequent calls, allowing the rep to pick up exactly where they left off, reinforcing the discussion and moving the customer along the adoption ladder. The brand team can then run reports on this aggregated CRM data to gain critical market and customer insights, informing and enhancing future marketing strategies. Key Takeaways: * **Functional Content Definition:** Functional content goes beyond static "digital paper" or basic interactive elements. It actively calculates, measures, records, and personalizes content, making it "work for a living" to achieve specific sales and marketing objectives. * **Enhanced Sales Effectiveness:** Implementing functional content significantly improves customer engagement, provides actionable market insights, monitors content impact, and ultimately leads to better sales outcomes by tailoring interactions to individual customer needs. * **Veeva CLM as a Platform:** Veeva CLM multi-channel presentations (eDetailers, eSales Aids) are ideal vehicles for deploying functional content, allowing for dynamic and data-driven sales interactions within a regulated environment. * **Empowering Key Account Managers (KAMs):** Functional content equips KAMs to effectively set up sales calls, deeply understand customer needs through interactive engagement, facilitate strong closes with personalized commitments, and seamlessly set up follow-up discussions. * **Data Capture and CRM Integration:** A critical aspect is the ability of functional content to capture customer-specific data (e.g., patient prioritization, treatment percentages) and save it directly against the customer's record within the Veeva CRM. * **Personalized Content Delivery:** The data collected through functional content can dynamically drive and personalize subsequent parts of the presentation, ensuring that the information presented is most relevant and impactful for the individual healthcare professional. * **Driving Future Interactions:** Stored customer data can be used to power content for subsequent sales calls, allowing reps to resume conversations from where they left off, track progress, and reinforce commitments, effectively guiding customers along the adoption ladder. * **Valuable Market and Customer Insights:** The aggregated data from functional content across the sales force provides brand teams with critical market and customer insights, which are invaluable for refining marketing plans, identifying trends, and optimizing strategies. * **Strategic Closing Techniques:** Functional content enables KAMs to craft highly personalized closing statements based on the customer's input during the presentation, leading to more concrete commitments and measurable outcomes. * **Multifunctional Design:** A single piece of well-designed functional content can serve multiple purposes within a sales call, from initial needs assessment to closing and even setting the stage for future engagements. **Tools/Resources Mentioned:** * Veeva CRM * Veeva CLM (Multichannel Presentations, eDetailers, eSales Aids, Digital Sales Aids) **Key Concepts:** * **Functional Content:** Content designed to actively engage users and customers by calculating, measuring, recording, and personalizing information for maximum impact and specific outcomes. * **Veeva CLM:** Content Lifecycle Management, a module within Veeva CRM, used for creating, managing, and delivering interactive sales presentations and eDetailers to healthcare professionals. * **Critical Success Factor (CSF):** A key objective or goal for a brand or campaign, often used to measure success (e.g., increasing recognition of a new drug indication). * **Adoption Ladder:** A conceptual model illustrating the stages a customer progresses through from initial awareness to full adoption and advocacy of a product or behavior.

28b Veeva Insights - Episode 1: Veeva CRM Approved Email
twentyeightb
/@twentyeightb
May 13, 2019
This video provides an in-depth exploration of Veeva CRM Approved Email (VAE), a critical tool within the Veeva CRM platform designed to empower pharmaceutical brand teams and field specialists. The presenter, James Harper, founder of twentyeightb, introduces VAE as an essential component for leveraging multi-channel marketing efforts and enhancing customer engagement. The primary objective of the episode is to guide users through the functionalities of VAE, particularly focusing on its use within the CRM for iPad application (formerly iRep), to send compliant, approved, and personalized content to healthcare professionals and contacts. The discussion highlights VAE's integral role within the broader Veeva ecosystem, emphasizing its seamless integration with other CRM and CLM tools such as Veeva Engage and Veeva Vault documents. A key advantage presented is the robust analytics capability, where VAE usage data, including send and open rates, feeds directly back into the CRM system. These analytics are crucial for motivating field teams, increasing platform adoption, measuring and enhancing sales effectiveness, and providing valuable market insights back to brand or medical teams. The video details various practical applications for VAE, such as following up on calls, sharing educational materials, distributing budget impact models, and inviting customers to webinars or remote detail sessions. The core of the episode walks viewers through the classic VAE user interface on the CRM for iPad. This includes selecting a target customer, accessing the "send email" action, and navigating the highly granular control system that ensures only appropriate and approved content is available for specific customers. The process involves selecting an email template, which forms the base structure with greetings, disclaimers, and corporate branding. Users then have the ability to personalize the email by adding "fragments"—small, approved content elements. The video demonstrates how personalization can be managed through drop-down lists and free-text fields, with built-in controls like blacklisting words and mandating field completion to maintain compliance. A notable feature is the automated referencing system, which intelligently renumbers references even when fragments are reordered, ensuring accuracy. The episode concludes with a demonstration of previewing the email and the "slide-to-send" mechanism, a thoughtful design choice to prevent accidental dispatches. Key Takeaways: * **Compliant Personalized Communication:** Veeva Approved Email (VAE) enables pharmaceutical field teams to send highly personalized email content to customers and contacts while strictly adhering to regulatory and brand compliance standards. * **Integrated Veeva Ecosystem:** VAE is an integral part of the Veeva platform, seamlessly integrating with other critical tools like Veeva CRM, CLM (Content Lifecycle Management), Veeva Engage, and Veeva Vault documents, ensuring a unified multi-channel marketing approach. * **Actionable Analytics for Performance:** Usage analytics, including send and open rates, are automatically fed back into the CRM system, providing detailed insights that can be used to motivate field teams, increase platform usage, enhance sales and customer engagement effectiveness, and generate market insights for brand and medical teams. * **Diverse Use Cases:** VAE supports a wide range of commercial operations, including post-call follow-ups, sharing educational materials, distributing budget impact models, and sending invitations to educational meetings, webinars, or remote detailing sessions. * **Granular Content Control:** The platform offers a highly granular level of control over email templates and content fragments, ensuring that only content appropriate and approved for specific users and target customers is available for distribution. * **Robust Personalization Features:** Users can personalize emails through various mechanisms, including drop-down lists and free-text fields, with built-in compliance features such as blacklisting specific words or terms and mandating field completion before an email can be sent. * **Automated Referencing System:** A clever feature of VAE is its automated referencing system, which automatically renumbers references within an email even when content fragments are reordered, maintaining accuracy and compliance without manual intervention. * **Intuitive User Interface:** The video demonstrates a straightforward user interface within the CRM for iPad app, guiding users through selecting customers, choosing templates, adding and reordering content fragments, and previewing the final email. * **"Slide-to-Send" Mechanism:** To prevent accidental email dispatches, VAE incorporates a "slide-to-send" icon, requiring a deliberate user action to confirm sending, enhancing user control and reducing errors. * **Strategic Multi-channel Enabler:** VAE is positioned as an essential tool for field specialists to link up and fully utilize the suite of multi-channel marketing tools and materials available to them, bridging the gap between in-person interactions and digital engagement. * **Future Enhancements:** The series promises to cover advanced functionalities in subsequent episodes, such as launching VAE directly from within a detail aid and leveraging Veeva CRM MyInsights dashboards to inform and enhance a user's effectiveness in the field. Tools/Resources Mentioned: * Veeva CRM * Veeva Approved Email (VAE) * CRM for iPad (previously known as iRep) * Veeva CLM (Content Lifecycle Management) * Veeva Engage * Veeva Vault documents * Veeva CRM MyInsights (mentioned as a topic for future episodes) Key Concepts: * **Veeva Approved Email (VAE):** A core module within Veeva CRM designed to enable pharmaceutical field teams to send pre-approved, compliant, and personalized email communications to healthcare professionals and other contacts. It ensures all outbound email content adheres to regulatory guidelines and brand messaging. * **Email Template:** The foundational structure of an email within VAE, which typically includes standard greetings, corporate logos, disclaimers, and sign-offs. These templates provide a consistent and compliant framework for all communications. * **Fragments:** Modular, pre-approved content blocks that users can select and insert into an email template. Fragments allow for dynamic personalization and ensure that even customized emails remain compliant with pre-defined messaging and regulatory requirements. * **Automated Referencing System:** A sophisticated feature of VAE that automatically manages and renumbers references within an email as fragments are added, removed, or reordered. This ensures that all scientific and medical claims are accurately sourced and compliant, even with dynamic content changes. * **Multi-channel Marketing:** A strategic approach that integrates various communication channels (e.g., email, in-person visits, webinars, digital content) to engage customers effectively. VAE is presented as a key tool for connecting these channels within the pharmaceutical commercial strategy.

28b Veeva Insights - Episode 2: Advanced Veeva CRM Approved Email
twentyeightb
/@twentyeightb
May 13, 2019
This video provides an in-depth exploration of advanced implementations of Veeva CRM Approved Email (VAAIE) and the strategic utilization of MyInsights dashboards to enhance the effectiveness of Key Account Managers (KAMs) in the field. James Harper, Founder and Managing Director of 28b, guides brand teams and creative agencies through leveraging these Veeva CRM tools, focusing on practical applications for optimizing commercial operations. The episode builds upon foundational knowledge of VAAIE, delving into more sophisticated integrations and data visualization techniques. The presentation begins by demonstrating how KAMs can seamlessly launch Approved Emails directly from within an eDetail. This streamlined process is exemplified by a scenario where a new product guideline necessitates informing customers and inviting them to local medical education meetings. The video illustrates how a KAM can engage a customer with the meeting idea, navigate to a dedicated "meetings tab" within the eDetail, select a suitable time and location, and then, with a single tap, launch a pre-populated VAAIE template. This integration significantly reduces the time and effort required to send targeted communications, allowing KAMs to quickly return to their eDetail and continue their sales conversation. Following the demonstration of efficient email delivery, the video transitions to the critical role of Veeva CRM MyInsights in maximizing the return on investment (ROI) for these medical education meetings. MyInsights is presented as an innovative data visualization capability designed to deliver relevant information precisely when and where field teams need it. The speaker showcases a custom-built MyInsights dashboard accessible at the territory level, providing KAMs with a quick overview of recruitment progress across multiple meetings. This dashboard integrates standard VAAIE analytics—such as email sent, opened, and link clicked—with data from Veeva CRM Events Management to track actual event registrations. By visually representing recruitment status and identifying specific customers to follow up with, MyInsights empowers KAMs with actionable intelligence to ensure maximum attendance and optimal ROI for their initiatives. Key Takeaways: * **Integrated Approved Email Launch:** Veeva CRM Approved Email (VAAIE) can be launched directly from within an eDetail, significantly streamlining the communication process for Key Account Managers (KAMs) and enabling them to send targeted emails in seconds. * **Enhanced Field Efficiency:** This direct launch capability allows KAMs to quickly send critical information, such as invitations to medical education meetings, without leaving their eDetail, thus minimizing disruption and maximizing time spent on selling. * **Automated Template Population:** The system can automatically select the correct VAAIE template and populate it with relevant fragments (e.g., meeting details), reducing manual effort and ensuring consistency and compliance. * **Strategic Use of Veeva CRM MyInsights:** MyInsights is a powerful data visualization tool within Veeva CRM that provides field teams with timely and relevant information, accessible at both customer and territory levels. * **Optimizing Medical Education Meetings:** Custom MyInsights dashboards can be designed to specifically track and optimize the recruitment for local medical education meetings, providing KAMs with a clear overview of their progress. * **Actionable Recruitment Insights:** These dashboards combine standard VAAIE analytics (email sent, opened, clicked) with Veeva CRM Events Management data (event registration) to give KAMs a comprehensive view of customer engagement and attendance. * **Data-Driven Follow-up:** MyInsights empowers KAMs with actionable information, visually indicating recruitment performance and identifying specific customers who should be invited next or require follow-up, ensuring maximum attendance and ROI. * **Focus on Sales Effectiveness:** The ultimate goal of these advanced Veeva CRM implementations is to enhance infield sales effectiveness and contribute to positive sales outcomes by providing field teams with the tools and insights they need. * **Future Content Integration:** The video hints at future episodes that will explore functional eDetail content, market insights, KPI tracking, and monitoring content usage, all aimed at further improving sales effectiveness. Tools/Resources Mentioned: * Veeva CRM * Veeva CRM Approved Email (VAAIE) * Veeva CRM MyInsights * Veeva CRM Events Management Key Concepts: * **eDetail:** Electronic detailing, an interactive presentation used by sales representatives on tablets or other devices to engage healthcare professionals. * **Key Account Manager (KAM):** A sales role focused on managing and developing relationships with strategically important customers or accounts. * **Approved Email:** A compliant email communication system within Veeva CRM that ensures all content is pre-approved by regulatory and medical teams before being sent to healthcare professionals. * **MyInsights:** A data visualization and dashboarding capability within Veeva CRM that allows for custom reports and actionable insights to be delivered directly to field teams. * **Medical Education Meetings:** Events organized by pharmaceutical companies to educate healthcare professionals on new products, guidelines, or therapeutic areas. * **ROI (Return on Investment):** A performance measure used to evaluate the efficiency of an investment or to compare the efficiency of several different investments. In this context, it refers to the effectiveness of medical education meetings in terms of customer engagement and attendance.
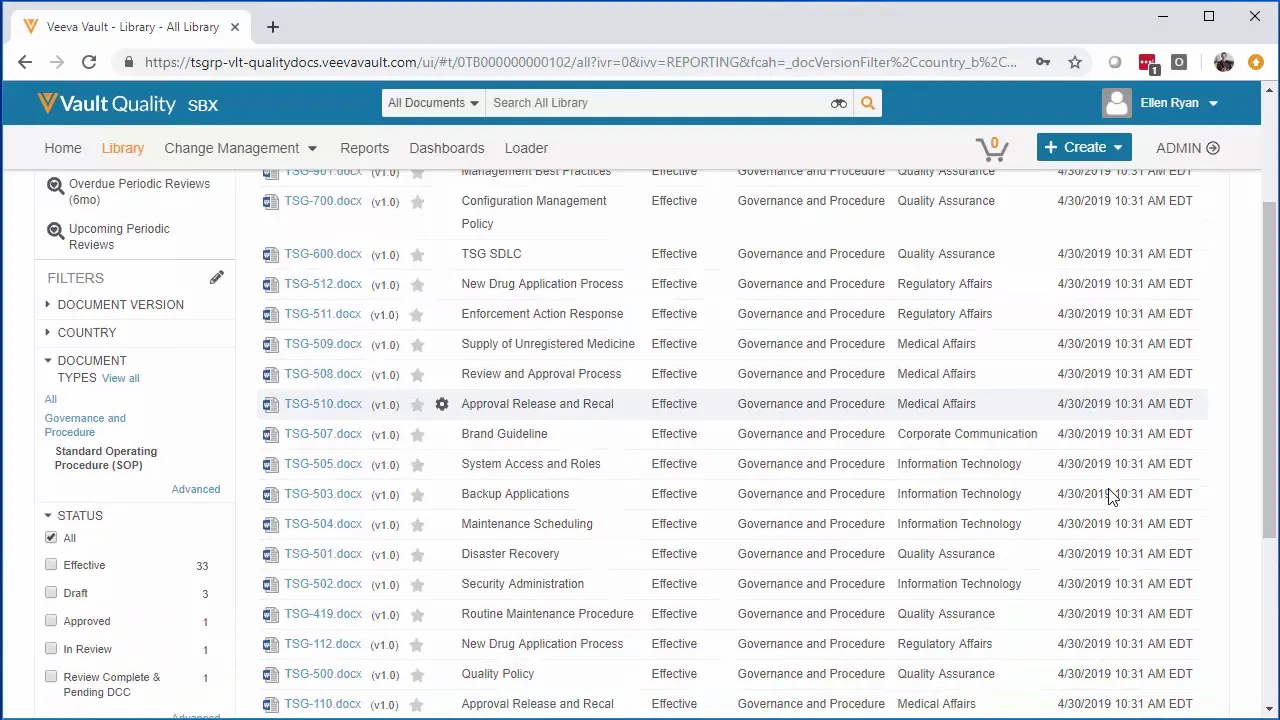
Veeva Vault - Ongoing Migrations
Technology Services Group is now part of Hyland
/@tsgrp
Apr 30, 2019
This video provides a practical demonstration of managing content synchronization during a phased rollout of a new Veeva Vault content management system. The primary focus is on utilizing the OpenMigrate tool to handle ongoing data migrations, ensuring continuity and data integrity when users are onboarded to the new platform in segments rather than through a "Big Bang" approach. The scenario addresses the common challenge of maintaining operational efficiency during the transition period when multiple systems must operate in tandem. The speaker establishes a realistic scenario often encountered by pharmaceutical and life sciences clients transitioning to Veeva Vault. This involves a transitional period where certain departments, specifically Regulatory Affairs and Quality Assurance, have already been migrated to Veeva Vault, while others, such as Medical Affairs, Information Technology, and Corporate Communications, remain temporarily on the legacy system (identified as Documentum/Webtop). The critical necessity highlighted is maintaining visibility and consistency across both platforms. Users already operating on Veeva Vault must be able to access documents created by users still operating in the legacy environment, supporting essential cross-functional workflows. The demonstration illustrates the mechanism for continuous synchronization facilitated by OpenMigrate. While an initial migration handles the bulk content load, the ongoing migration process addresses new content or updates made in the legacy system after the initial cutover. By scheduling the migration job (e.g., hourly or daily), the system automatically ingests and synchronizes new content, preventing data from being "left stranded" in the old system. The speaker illustrates this by adding a new document under "Corporate Communications" in the legacy Webtop interface and then showing its subsequent appearance and synchronization within Veeva Vault following the scheduled OpenMigrate run. This methodology is positioned as essential for minimizing disruption and ensuring a smooth, segmented onboarding process for various departments. The core value proposition of this approach is enabling a smooth, phased transition that accommodates organizational complexity and regulatory requirements. Instead of forcing all departments to switch simultaneously, which often leads to operational bottlenecks and compliance risks, the use of continuous migration allows for a controlled, risk-mitigated rollout. This strategy ensures that even during the transition period—which can last days, weeks, or months—all users, regardless of their current platform, have access to the most up-to-date content and metadata, supporting ongoing business operations and maintaining audit readiness. Key Takeaways: * **Phased Rollout Strategy:** The video strongly advocates for a segmented migration strategy for Veeva Vault adoption, rolling out the platform by department (e.g., Regulatory Affairs and Quality Assurance first) or by document type, as opposed to a high-risk, disruptive "Big Bang" approach. * **Managing Dual-System Operations:** Successful migration planning must account for the inevitable transitional period—potentially lasting weeks or months—where both the legacy content management system and the new Veeva Vault platform must be used concurrently by different user groups. * **Continuous Synchronization Requirement:** To maintain data integrity and operational continuity, content created or updated in the legacy system after the initial bulk migration must be continuously synchronized with Veeva Vault using scheduled jobs (e.g., hourly or daily). * **Preventing Stranded Content:** Without a continuous migration process, any new content added to the legacy system after the initial migration is completed risks being "stranded," creating significant data gaps and accessibility issues for users on the new platform. * **Cross-Departmental Visibility:** The migration solution must ensure that users who have already transitioned to Veeva Vault can seamlessly access and view documents originating from departments still operating on the legacy system, supporting essential cross-functional workflows like those between Regulatory and Corporate Communications. * **OpenMigrate as the Synchronization Engine:** The demonstration specifically identifies OpenMigrate as the tool leveraged to execute the scheduled, ongoing migrations, highlighting its capability to handle the delta (new and updated content) between the two systems. * **Scope of Data Transfer:** Effective synchronization requires the transfer of both the content files themselves and the associated metadata from the legacy system (e.g., Documentum/Webtop) to ensure proper categorization and searchability within Veeva Vault. * **Minimizing Operational Disruption:** The phased approach, supported by continuous migration, minimizes disruption to critical business processes, allowing departments to onboard at a sustainable pace without compromising access to essential compliance or commercial documents. * **Post-Initial Load Focus:** After the initial data load, the focus of the migration effort shifts entirely to managing the delta—ensuring that all changes and new creations in the legacy system are rapidly reflected in Veeva Vault to establish it as the definitive single source of truth. * **Regulatory Compliance Support:** For highly regulated environments, the ability to maintain a consistent, synchronized view of controlled documents across platforms during the transition is crucial for maintaining GxP compliance and audit readiness. Tools/Resources Mentioned: * **Veeva Vault:** The target content management platform, commonly used in life sciences for regulated content (e.g., Quality, Regulatory, Clinical). * **OpenMigrate:** The specific tool demonstrated for executing both initial bulk migrations and ongoing, scheduled content synchronization between systems. * **Documentum/Webtop:** The legacy content management system referenced as the source platform for the migration. Key Concepts: * **Phased Rollout:** A strategy for implementing a new enterprise system where different user groups or functionalities are introduced sequentially over time, rather than all at once. * **Big Bang Migration:** A high-risk migration strategy where all users and data switch from the legacy system to the new system simultaneously on a single cutover date. * **Ongoing Migration/Synchronization:** The process of continuously transferring new or updated content from a legacy system to a new system after the initial bulk load, ensuring data consistency during a prolonged transition period. * **Stranded Content:** Content created in a legacy system after the official migration cutover that is not transferred to the new system, making it inaccessible to users on the new platform.

Customer Success Story | Regulatory Affairs Professionals Society (RAPS)
Higher Logic
/@HigherLogic
Apr 29, 2019
This video presents a customer success story detailing how the Regulatory Affairs Professionals Society (RAPS) leveraged community engagement and marketing automation tools from Higher Logic to better serve its growing, global membership base. The primary goal of RAPS, as stated in the video, is to support regulatory professionals in their mission to create an atmosphere of better public health for the future. The organization recognized that as it expanded both in membership size and global reach, it needed a centralized, robust platform to maintain member connection and deliver value. The core strategy implemented by RAPS was the establishment of the "Regulatory Exchange" community, which is positioned as the central hub of the entire organization. A critical investment supporting this initiative was the hiring of a full-time Higher Logic Community Manager, which RAPS identifies as one of the best investments made in their community infrastructure. This focus on dedicated management has paid dividends, resulting in the community now being ranked as one of RAPS' most highly used and valuable member benefits. This success underscores the principle that technology adoption must be paired with dedicated human resources for optimal engagement and value delivery. Beyond community engagement, RAPS also implemented marketing automation to streamline internal processes and gain deeper insights into member needs. In the association world, retention is paramount, and RAPS observes a direct correlation between engagement and renewal rates. Specifically, they noted that volunteers—who are highly engaged members—show continuously increasing renewal numbers because they feel a strong sense of community and belonging. Marketing automation has transformed the department's ability to listen to customers, making the connection between what members need and what RAPS produces a tangible reality. This capability allows RAPS to dynamically tailor its offerings and communications, ensuring the organization remains relevant and valuable to its specialized audience of regulatory affairs professionals. Key Takeaways: * **Centralized Community Strategy:** RAPS successfully positioned its online platform, the "Regulatory Exchange," as the central hub for the organization, essential for managing a growing and globally expanding membership base within the highly specialized regulatory affairs sector. * **Investment in Dedicated Management:** A crucial factor in the community's success was the investment in a full-time Higher Logic Community Manager, highlighting that technology platforms require dedicated human oversight and expertise to maximize member engagement and platform utility. * **Engagement Drives Retention:** The video reinforces the well-documented association principle that a more engaged member is significantly more likely to renew their membership. RAPS specifically tracks increasing renewal rates among its highly engaged volunteer base, confirming the value of fostering a sense of belonging. * **Community as a Top Member Benefit:** The Regulatory Exchange community has become one of RAPS' most highly used and valuable member benefits, demonstrating the necessity of providing peer-to-peer interaction and resource sharing for professionals in complex fields like regulatory affairs. * **Marketing Automation for Insight:** Implementation of marketing automation has streamlined RAPS’ internal marketing processes and, more importantly, made "listening to our customers" a reality. This technology enables the organization to connect member needs and wants directly to the content and services it produces. * **Connecting Needs to Output:** The ability to link customer demands (regulatory professionals' specific challenges) with organizational output (training, resources, networking) is a key outcome of the integrated community and automation strategy, ensuring RAPS' offerings remain relevant and timely. * **Supporting Public Health Mission:** The underlying mission of RAPS—supporting regulatory professionals in creating better public health—is directly supported by these engagement tools, as they facilitate the rapid sharing of information and best practices among the professional community. * **Value Proposition for Specialized Audiences:** For organizations serving highly specialized, regulated fields (like life sciences), providing a dedicated, managed community space is critical for knowledge exchange, professional development, and maintaining organizational relevance. Tools/Resources Mentioned: * **Higher Logic:** The platform utilized by RAPS for both community management and marketing automation. * **Regulatory Exchange:** The specific online community platform established by RAPS for its members. Key Concepts: * **Member Engagement:** The active participation and interaction of members within the association, directly correlated with member satisfaction and retention rates. * **Marketing Automation:** Software that automates repetitive marketing tasks (like email campaigns, segmentation, and data tracking) to improve efficiency and gain deeper insights into customer behavior. * **Regulatory Professionals:** Individuals responsible for ensuring that pharmaceutical, biotech, and medical device products comply with government regulations (e.g., FDA, EMA) throughout the development and commercial lifecycle.

RealTime Site Operations Management System (SOMS)
RealTime eClinical Solutions
/@RealTime-eClinical
Apr 25, 2019
The video introduces the RealTime Site Operations Management System (SOMS), positioning it as the most comprehensive and powerful solution developed for clinical research sites and site networks. The core value proposition of SOMS is the integration of multiple, proven standalone solutions into a single, customizable, and user-friendly interface designed to manage all facets of site operations efficiently. This integrated approach aims to help research sites achieve "peak performance" by consolidating disparate functions typically managed across multiple systems. The foundation of the SOMS is the RealTime Clinical Trial Management System (CTMS), which offers extensive features spanning both commercial and operational aspects of clinical trials. On the commercial side, the CTMS includes sophisticated recruitment modules that facilitate marketing campaign management, website and social platform integration, email client integration, lead tracking, and call center management features. Crucially, it also incorporates automated and direct-to-patient text messaging capabilities and robust campaign result reporting, allowing sites to optimize their patient acquisition strategies. Operationally, the CTMS manages essential functions such as subject visits, study financials (invoicing, collections, and payables), and scheduling, complete with Outlook integration. The true power of the RealTime SOMS lies in its ability to seamlessly integrate optional, complete systems directly into the CTMS interface. These specialized modules address critical areas of clinical trial execution and compliance. Specifically, the system offers RealTime Pay for streamlined subject payments, RealTime Text for sending patients timely alerts regarding upcoming appointments, and RealTime eReg for comprehensive electronic regulatory management. Perhaps most critically for data integrity and compliance, the system includes RealTime eSource, which enables sites to collect data electronically. This feature is highlighted as essential for ensuring complete, accurate, and compliant data collection, while also providing real-time access to study data for sponsors and Contract Research Organizations (CROs). The overall message emphasizes that this unified system simplifies complex clinical operations, driving efficiency and regulatory adherence. Key Takeaways: • **Integrated Site Operations Management:** The RealTime SOMS is presented as a unified platform that bundles previously standalone solutions (CTMS, eReg, eSource, Payments) into a single interface, eliminating data silos and improving operational efficiency for clinical research sites and site networks. • **Comprehensive Recruitment and Marketing Tools:** The CTMS component features advanced recruitment modules, including capabilities for managing marketing campaigns, integrating with social platforms and websites, tracking leads, and utilizing call center management features, which are vital for optimizing patient enrollment. • **Automated Patient Communication:** The system leverages automated and direct-to-patient text messaging (via RealTime Text) for appointment alerts and communication, significantly reducing no-show rates and improving patient engagement throughout the trial lifecycle. • **Financial and Administrative Streamlining:** Core administrative functions are managed within the CTMS, including the tracking of subject visits, comprehensive study financials (invoicing, collections, and payables), and scheduling with integrated Outlook functionality. • **Electronic Regulatory Compliance (eReg):** The RealTime eReg module provides complete electronic management of regulatory documentation, which is crucial for maintaining audit readiness and adhering to complex clinical trial regulations. • **Compliant Electronic Source Data (eSource):** The RealTime eSource system allows for electronic data collection directly at the site, ensuring that data is complete, accurate, and compliant, thereby addressing critical requirements for GxP and regulatory standards. • **Real-Time Data Access for Sponsors/CROs:** The system facilitates enhanced transparency by providing sponsors and CROs with real-time access to study data, enabling faster decision-making and oversight, which is a key requirement for modern decentralized clinical trials. • **Opportunity for AI/Automation Integration:** The consolidation of recruitment data, financial data, regulatory documents, and eSource data into one system creates a robust, centralized data foundation, making it an ideal candidate for AI and LLM solutions aimed at predictive analytics, automated reporting, and intelligent workflow optimization. Tools/Resources Mentioned: * RealTime Site Operations Management System (SOMS) * RealTime CTMS (Clinical Trial Management System) * RealTime Pay (Subject Payment System) * RealTime Text (Patient Alert System) * RealTime eReg (Electronic Regulatory Management System) * RealTime eSource (Electronic Source Data Collection System) * Outlook (Integration for scheduling) Key Concepts: * **Site Operations Management System (SOMS):** A holistic platform designed to manage all administrative, financial, regulatory, and patient-facing activities required to run a clinical research site efficiently. * **CTMS (Clinical Trial Management System):** Software used by pharmaceutical companies and clinical research sites to manage and track the operational aspects of a clinical trial, including subject enrollment, milestones, budgets, and deadlines. * **eSource:** The electronic capture of source data (the original data recorded in a clinical trial) directly into an electronic system, ensuring data quality and compliance while eliminating the need for paper records. * **eReg:** The electronic management of essential regulatory documents required for clinical trials, replacing traditional paper binders and streamlining the audit process.
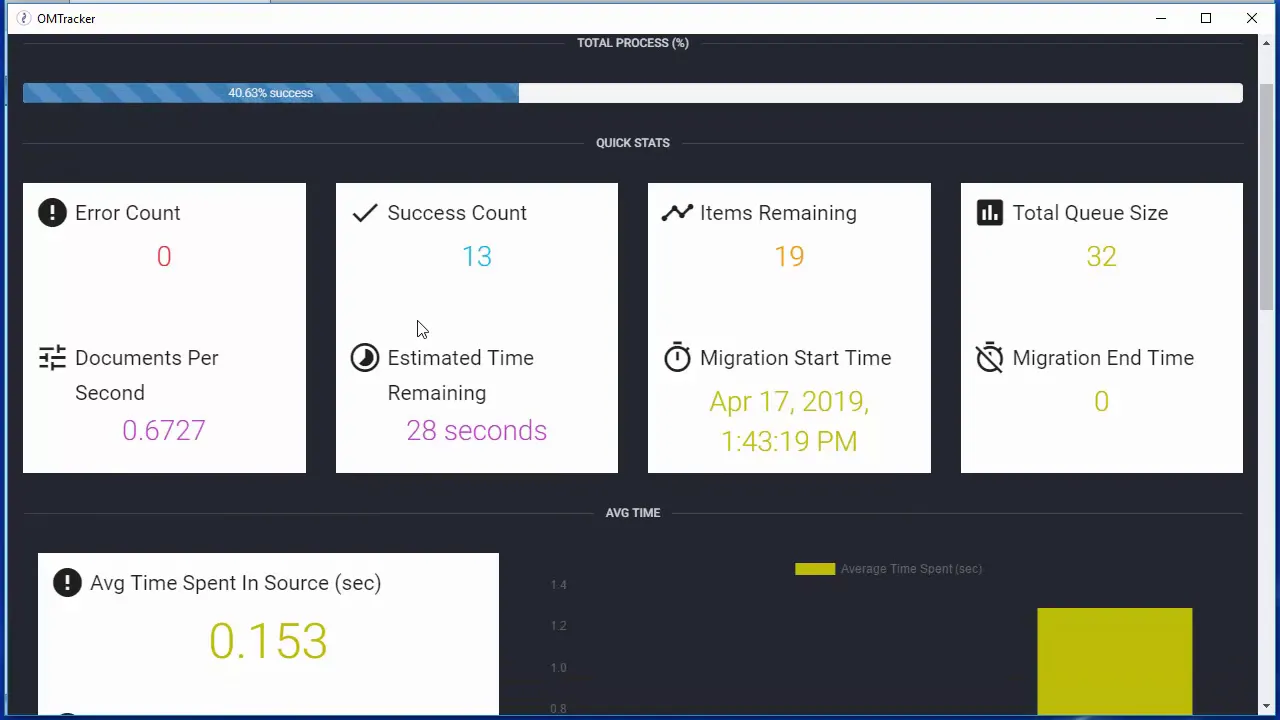
Documentum to Veeva Vault - Migration for Life Sciences
Technology Services Group is now part of Hyland
/@tsgrp
Apr 17, 2019
The video demonstrates a technical solution for migrating regulated content from legacy Enterprise Content Management (ECM) systems, specifically Documentum (Webtop), into Veeva Vault QMS. The core focus is on utilizing a specialized tool, OpenMigrate, enhanced with a Veeva Target connector, to execute an automated, single-step migration. This approach is highlighted as a critical benefit for regulated life science companies because it significantly streamlines the validation efforts required for system transitions and eliminates the risks associated with manual intervention. The presentation walks through the configuration of the migration, setting the legacy system as the source and Veeva Vault as the target, emphasizing the capability for complex data harmonization during the transfer. A crucial element of the demonstration is the real-time monitoring of the migration process using the OM Tracker dashboard. This tool provides operational transparency by displaying key metrics such as server memory utilization, documents successfully migrated, error counts, remaining queue size, and detailed timing statistics (e.g., average time spent in the source versus the target system). This level of granular monitoring is essential for managing the performance and integrity of large-scale content transfers in a regulated environment. Furthermore, the video stresses the importance of metadata mapping, illustrating how OpenMigrate can translate complex source attributes—such as mapping an abbreviated department acronym in Documentum to its full, unabbreviated format in Veeva Vault—to ensure data consistency and compliance with the target system’s data model. Upon completion, the migration generates a success log in an Excel spreadsheet format. This log is presented as a vital component of the validation package, providing an auditable record with a row for every successfully transferred document, alongside all its associated metadata from the source system. The final step involves verifying the content within the target system, Veeva Vault QMS, confirming that the migrated Standard Operating Procedures (SOPs) are visible and that the metadata mapping, particularly the department attribute transformation, was executed correctly. The presenter concludes by assuring viewers that this methodology is adaptable, supporting various source systems including D2 and standard file systems, ensuring a direct and compliant path to Veeva Vault. Key Takeaways: * **Streamlined Validation through Automated Migration:** The use of a specialized tool like OpenMigrate allows for a single-step migration directly into Veeva Vault, which drastically reduces the complexity of validation and compliance documentation required for regulated content transfers in the life sciences sector. * **Mandatory Real-Time Performance Monitoring:** Tools like OM Tracker are essential for managing regulated migrations, providing critical metrics such as error rates, throughput, and queue sizes, enabling proactive intervention to maintain data integrity and project timelines. * **Complex Metadata Mapping is Non-Negotiable:** Successful migration requires the ability to transform and harmonize metadata on the fly. The demonstration highlights mapping abbreviated legacy attributes (e.g., department acronyms) to full, compliant values in Veeva Vault, which can be configured internally or via external lookup tables/databases. * **Audit-Ready Success Logs are Critical:** The migration process must generate a comprehensive, auditable success log (e.g., an Excel spreadsheet) detailing every transferred document and its source metadata. This log serves as the primary evidence required for regulatory validation and audit trails. * **Veeva Vault QMS as the Target Standard:** The focus on migrating SOPs into Veeva Vault QMS underscores the industry trend toward utilizing specialized, cloud-based platforms for managing GxP-critical documentation and quality processes. * **Source System Flexibility:** The migration solution is versatile, capable of handling content not only from traditional ECM systems like Documentum (Webtop, D2) but also from basic file systems, offering a unified approach for companies with heterogeneous content storage environments. * **Focus on Regulated Document Types:** The migration of Standard Operating Procedures (SOPs) emphasizes the solution’s application to GxP documentation, where maintaining version history, integrity, and compliance during system transition is paramount. * **Timing Metrics for Optimization:** The OM Tracker provides detailed timing metrics (time spent in source processing vs. time spent in target ingestion), which are valuable for identifying performance bottlenecks and optimizing the migration configuration for large datasets. Tools/Resources Mentioned: * **OpenMigrate:** The migration software platform. * **OpenMigrate Veeva Target Connector:** The specific module enabling migration into Veeva Vault. * **OM Tracker:** A proprietary real-time migration monitoring dashboard. * **Documentum (Webtop, D2, DCM):** Legacy source ECM systems. * **Veeva Vault QMS:** The target Quality Management System platform. Key Concepts: * **One-Step Migration:** A direct transfer method designed to minimize validation steps and complexity compared to multi-stage migrations. * **Metadata Mapping:** The process of translating data fields between two different system schemas to ensure data consistency and accuracy in the target system. * **Validation Log:** An exhaustive record of successful transfers and associated metadata, required for demonstrating regulatory compliance post-migration.

eTMF Working Paperless
ECCRT
/@eccrt1731
Apr 5, 2019
This video explores the critical transition to eTMF (electronic Trial Master File) and paperless operations within clinical research, focusing on regulatory requirements and practical implementation. The speaker, Dr. Marleen Van Bael, details the advantages of digital documentation, outlines the latest guidelines from the European Medicines Agency (EMA) and Good Clinical Practice (GCP), and provides insights into ensuring compliance when working with electronic trial documents. The discussion covers the necessity of validated computerized systems, the process of converting paper to digital, and the evolving landscape of electronic source data. Key Takeaways: * **Regulatory Mandate for Digital:** EMA and GCP guidelines require robust electronic systems for trial documentation, treating electronic and paper formats with equal stringency, emphasizing the industry's shift towards digital. * **Multifaceted Benefits of Paperless:** Beyond mere efficiency, electronic systems offer enhanced data quality, immediate access to up-to-date information, improved traceability, support for risk-based monitoring and remote work, and greater protection against physical data loss. * **Validation is Non-Negotiable:** Computerized systems for eTMF and electronic source data must be thoroughly validated to guarantee data integrity, security, comprehensive audit trails (tracking all actions), and protection against any unauthorized alterations, adhering strictly to GxP principles. * **Certified Copies and Secure Destruction:** An electronic "certified copy" requires a validated system that ensures it's an exact replica of the original. Paper originals can only be destroyed if the electronic version is created via a validated process with stringent quality control during scanning, ensuring no data is lost or altered. * **Operational Preparedness is Key:** Successful adoption of paperless systems necessitates clear Standard Operating Procedures (SOPs), consistent file naming conventions, role-based access controls, and mandatory training for all personnel involved in managing electronic documentation. * **Rise of Electronic Source Data:** The industry is increasingly moving towards direct electronic source data capture (eSource), with EMA providing guidance on expectations for such systems, highlighting the need for robust integration and control mechanisms, often involving hospital-owned data.

Veeva CEO: Revolution in Medicine | Mad Money | CNBC
CNBC Television
/@CNBCtelevision
Mar 12, 2019
This video features an interview with Peter Gassner, CEO of Veeva Systems, on CNBC's Mad Money, where he discusses the company's significant growth and its pivotal role in the ongoing revolution in medicine. The conversation highlights Veeva's cloud-based software solutions for the life sciences industry, emphasizing how these tools enhance efficiency, ensure regulatory compliance, and support pharmaceutical companies in bringing new drugs to market. Gassner explains Veeva's journey from a niche player to a dominant force, particularly with the success of its Veeva Vault platform, and outlines the company's future trajectory amidst the emergence of precision medicine and the increasing integration of artificial intelligence in healthcare. The discussion delves into Veeva's value proposition, asserting that their cloud software not only saves individual customers money but also significantly increases the overall efficiency of the $1.7 trillion pharmaceutical business. Gassner elaborates on how Veeva's integrated platform addresses various needs, from capturing clinical trial data and managing government regulations to optimizing sales force effectiveness. He underscores the importance of a unified, cloud-based system for life sciences companies, which has driven the rapid adoption and expansion of Veeva's offerings, leading to the company surpassing its revenue targets and projecting a total addressable market exceeding $9 billion. A significant theme explored is the "revolution in medicine," characterized by the shift towards precision medicine, cell therapy, and gene therapy. Gassner positions Veeva as a crucial enabler of this transformation, stating that their technology is "the brains behind a lot of that stuff" when Jim Cramer brings up artificial intelligence in healthcare. The interview touches upon the increasing valuation of innovative biotech companies, citing the acquisition of Spark Therapeutics by Roche as an example of the premium placed on cutting-edge science in areas like gene therapy for hereditary conditions. Veeva's collaboration with both large pharmaceutical companies and smaller, innovative biotechs like Spark Therapeutics and Bluebird Bio further solidifies its role at the forefront of this medical paradigm shift. Key Takeaways: * **Veeva's Dominance in Life Sciences Cloud:** Veeva Systems has established itself as a leading provider of cloud-based software for the life sciences industry, offering a comprehensive platform that supports pharmaceutical companies from clinical trials to commercial operations and regulatory compliance. * **Efficiency and Cost Savings:** Veeva's solutions are presented as critical for increasing efficiency across the $1.7 trillion pharmaceutical sector, not only by saving individual customers money but also by streamlining complex processes involved in drug development and delivery. * **Veeva Vault's Success:** The Veeva Vault platform has been a significant growth driver, expanding from 5% to nearly 50% of the company's revenue, demonstrating the industry's demand for integrated, cloud-based applications for various operational needs. * **Addressing Industry Needs:** Veeva's software helps pharmaceutical companies capture clinical trial data, comply with government regulations, and enhance the effectiveness of their sales representatives, covering critical aspects of the drug lifecycle. * **Early Stages of Market Penetration:** Despite reaching over a billion dollars in sales, Veeva's CEO believes the company is still in the "very early days" of the industry cloud for life sciences, with a total addressable market estimated at over $9 billion and continuous product expansion. * **Enabling Precision Medicine:** Veeva is strategically positioned to support the "revolution in medicine" towards precision medicine, cell therapy, and gene therapy, which aim to provide highly targeted treatments for individuals. * **AI as a Core Component:** Veeva plays a significant role in the artificial intelligence component of modern healthcare, with its CEO confirming that Veeva's technology is "the brains behind a lot of that stuff," aligning with the vision of AI making healthcare more human. * **Innovation Drives Acquisitions:** The interview highlights how innovation in areas like precision medicine and gene therapy is driving significant acquisitions in the biotech sector, as larger pharmaceutical companies seek to integrate cutting-edge science. * **Support for Diverse Clients:** Veeva works with a broad spectrum of clients, from large pharmaceutical corporations to smaller, innovative biotechs, facilitating groundbreaking work in new therapeutic areas. * **Strategic Product Development:** The company's strategy involves continuously adding new products to its portfolio, ensuring it remains at the forefront of technological advancements and expanding its market reach within the life sciences ecosystem. Tools/Resources Mentioned: * Veeva Systems (cloud-based software) * Veeva Vault (specific product platform) * Salesforce.com (mentioned in context of Peter Gassner's background and partnership) * "Deep Medicine: How Artificial Intelligence can make Healthcare Human Again" by Dr. Eric Topol (book reference) Key Concepts: * **Cloud-based Software:** Delivery of software as a service over the internet, enabling scalability, accessibility, and efficiency for life sciences operations. * **Life Sciences Industry Cloud:** Specialized cloud platforms tailored to the unique regulatory, data, and operational requirements of pharmaceutical, biotech, and medical device companies. * **Precision Medicine:** An emerging approach to disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person. * **Cell Therapy & Gene Therapy:** Advanced therapeutic approaches that involve introducing new genetic material or cells into a patient's body to treat or prevent disease. * **Artificial Intelligence (AI) in Healthcare:** The application of AI technologies to improve various aspects of healthcare, from drug discovery and clinical trials to diagnostics and patient care. * **Regulatory Compliance:** Adherence to government regulations and industry standards, particularly critical in the highly regulated pharmaceutical and life sciences sectors. Examples/Case Studies: * **Spark Therapeutics Acquisition:** The acquisition of Spark Therapeutics, a biotech company focused on gene therapy for hereditary blindness, by Roche, illustrating the premium placed on innovation in precision medicine. * **Bluebird Bio:** Mentioned as another innovative biotech company working on new therapeutic approaches.

Jennifer Trundle Recommends Veeva R&D Summit
Veeva Systems Inc
@VeevaSystems
Mar 5, 2019
This video serves as a strong endorsement for the Veeva R&D Summit, positioning it as a premier software conference for leaders and experts within the life sciences industry. The speaker, Jennifer Trundle, highlights the event's unique structure, which successfully balances three critical components essential for technology adoption and strategic planning in regulated environments: the vendor’s future roadmap, practical customer case studies, and vital user community interaction. This combination ensures attendees receive both high-level strategic direction from Veeva Systems and actionable, real-world insights from their peers, making the conference exceptionally valuable for executives, IT professionals, and operational leaders in pharmaceutical and biotech companies. The core value proposition of the summit lies in its ability to bridge the gap between technological innovation and practical application. By presenting the "roadmap from Veeva," the event provides attendees with a clear view of upcoming features, platform enhancements, and strategic direction for the Veeva Vault suite, particularly in areas like Clinical, Regulatory, Quality, and Safety. For firms specializing in custom software and AI integration, understanding this roadmap is crucial for ensuring that bespoke solutions remain compatible, compliant, and optimized for future versions of the Veeva platform. The emphasis on customer case studies further grounds the discussion in reality, showcasing successful implementations, lessons learned, and best practices adopted by top pharma companies, emerging biotechs, and Contract Research Organizations (CROs). The scale and focus of the event underscore its importance within the regulated life sciences ecosystem. With over 1,400 attendees, including executives and experts, the summit acts as a major hub for discussing industry challenges, particularly those related to clinical data management, regulatory compliance (GxP, 21 CFR Part 11), and operational efficiency in R&D. The focus on "user community interaction" fosters an environment where attendees can network, share solutions to common data engineering and compliance hurdles, and validate the need for innovative technologies, such as AI and LLMs, to streamline complex R&D processes. This gathering confirms the ongoing need for specialized consulting and development services that can integrate advanced technologies seamlessly into the established Veeva ecosystem. Key Takeaways: • **Strategic Importance of the Veeva Roadmap:** The summit provides essential foresight into Veeva’s platform development, allowing consulting firms and life sciences companies to align their long-term IT strategies, custom software development cycles, and data pipeline architectures with future Veeva capabilities. • **Validation of Industry Pain Points:** The extensive sharing of customer case studies (over 65 breakout sessions) offers deep insight into the specific operational challenges faced by pharma, biotech, and CROs, particularly concerning clinical data management, quality assurance, and regulatory submissions. • **High-Value Target Audience Concentration:** The attendance of over 1,400 life sciences leaders and executives confirms the event as a critical gathering point for decision-makers in commercial operations, medical affairs, clinical operations, and regulatory compliance—all core target departments for specialized AI and software services. • **Demand for Integrated Solutions:** The focus on R&D best practices highlights the need for solutions that integrate seamlessly across the Veeva Vault suite (e.g., Clinical, Quality, Regulatory), requiring expertise in data engineering and system integration to maximize platform investment. • **Community-Driven Best Practices:** The emphasis on user community interaction underscores the value of peer-to-peer learning regarding successful technology adoption, providing valuable intelligence on which specific implementations or third-party tools are gaining traction within the regulated environment. • **Relevance to Regulatory Compliance:** Given the R&D focus, discussions inevitably center on maintaining GxP compliance, 21 CFR Part 11 adherence, and efficient audit trails, reinforcing the demand for AI solutions that automate and streamline these regulatory requirements. • **CRO and Biotech Engagement:** The strong presence of emerging biotechs and CROs indicates a significant market segment actively seeking scalable, compliant technology solutions, often requiring bespoke software or specialized consulting to manage rapid growth and complex trials. • **Software Conference Model Success:** The testimonial praises the balance achieved between vendor strategy, practical application, and networking, suggesting that future industry engagement strategies should emulate this model to deliver maximum value to highly technical and regulated audiences. **Tools/Resources Mentioned:** * Veeva R&D Summit * Veeva Systems Inc (Implied platform: Veeva Vault suite for R&D, including Clinical, Regulatory, Quality, and Safety applications) **Key Concepts:** * **Veeva Roadmap:** The planned future development, feature releases, and strategic direction for Veeva Systems’ software platforms, essential for long-term IT planning and integration efforts. * **User Community Interaction:** The process of networking and sharing knowledge among platform users, which helps establish industry best practices and identify common implementation challenges. * **Life Sciences Leaders:** Executives and decision-makers from pharmaceutical, biotech, and CRO organizations responsible for R&D, clinical operations, and regulatory strategy.

How can trial partners interact and collaborate?
Veeva Systems Inc
@VeevaSystems
Feb 26, 2019
This video, presented during a Veeva Systems event and featuring perspectives from major industry players like Janssen, Syneos Health, and IACT Health, addresses the critical and unsustainable operational crisis facing the clinical trial ecosystem. The core message is that the methodologies and processes that have governed clinical research for the last two decades are fundamentally obsolete and incapable of handling the current demands of pharmaceutical development. The speaker highlights a severe and growing disparity: the massive, increasing volume of new clinical studies that need to be conducted globally is colliding head-on with a "diminishing number of quality clinical research sites." This collision point—where the volume of studies fails to "gel" with the limited capacity of the research infrastructure—creates a systemic bottleneck that threatens the speed and efficiency of bringing new therapies to market. The current model relies heavily on a small, globally constrained network of sites, which are often overburdened and operate using outdated, manual, or fragmented systems. The implication is clear: the industry must abandon its reliance on legacy processes and embrace comprehensive digital transformation to optimize collaboration and resource utilization among trial partners. The necessity for improved interaction and collaboration among sponsors, CROs, and research sites is presented as the only viable path forward to maximize the efficiency of the scarce, high-quality site resources. This operational challenge directly points to the need for advanced technology solutions, including robust data engineering to integrate disparate systems, and AI/LLM tools capable of automating complex tasks like site feasibility assessment, patient enrollment forecasting, and regulatory documentation. By leveraging innovative technology, the life sciences sector can move toward a more scalable, resilient, and compliant clinical operations model that can effectively manage the current study volume without compromising research quality. Key Takeaways: * **Methodological Obsolescence:** The clinical trial industry is operating with methodologies that are 20 years old, rendering them incapable of efficiently managing the current high volume of global studies and leading to operational failure points. * **Critical Site Capacity Constraint:** There is a recognized and severe shortage of high-quality clinical research sites globally, creating an unsustainable bottleneck that limits the industry's ability to execute necessary trials quickly and effectively. * **Collaboration as a Necessity:** Enhanced interaction and collaboration among all trial partners (sponsors, CROs, and sites) are not optional but essential for maximizing the utilization and performance of the limited high-quality research sites available. * **Need for Operational Paradigm Shift:** The industry must move away from simply trying to force studies through the existing, small network of sites and instead adopt new operational models, such as decentralized trials (DCTs) and AI-driven site selection, to distribute the burden. * **Data Integration Imperative:** To address the site capacity crisis, sponsors and CROs require sophisticated data engineering services to integrate real-time performance data from sites, enabling proactive management and resource allocation. * **AI for Site Optimization:** The challenge of finding and maintaining "quality" sites necessitates the use of AI and predictive analytics to better assess site feasibility, forecast enrollment, and standardize operational workflows across the network. * **Focus on Quality over Volume:** The emphasis on the "diminishing number of *quality* clinical research sites" suggests that the industry needs solutions that enhance the operational quality and compliance of existing sites, rather than just seeking out new, potentially inexperienced centers. * **Regulatory Compliance in Modernization:** Any shift toward new methodologies and digital tools must maintain rigorous adherence to regulatory standards (e.g., FDA, GxP, 21 CFR Part 11), ensuring that efficiency gains do not compromise data integrity or patient safety. * **Veeva Ecosystem Optimization:** Given the context of the Veeva event, the solution involves maximizing the investment in regulated enterprise platforms like Veeva CRM and Clinical Operations suites, integrating them with custom AI agents for intelligent automation. Tools/Resources Mentioned: * Veeva Systems (Contextual platform for clinical and commercial operations) * Janssen (Pharmaceutical Sponsor) * Syneos Health (Contract Research Organization - CRO) * IACT Health (Clinical Research Site Network) Key Concepts: * **Clinical Site Constraint:** The systemic limitation imposed by the small, globally distributed number of high-quality research sites, which cannot keep pace with the increasing volume of clinical studies. * **Methodological Obsolescence:** The recognition that traditional, manual, and siloed approaches to trial execution are no longer viable in the modern, high-volume pharmaceutical R&D environment.

Investigate, Identify, and Address Compliance Issues with Cority's Enterprise Quality Management
Cority Software
/@CoritySoftware
Feb 13, 2019
This video provides an in-depth overview of the challenges associated with traditional, paper-based Quality Management (QM) systems and introduces Cority’s Enterprise Quality Management platform as a solution for regulated environments. The central theme is the necessity of transitioning from manual, error-prone processes—which lead to lost documents and siloed critical information—to automated, digital systems to effectively manage quality standards in today's complex supply chains. The presenter emphasizes that speed and automation are essential for Quality Managers to address the demanding regulatory landscape and complex global operations. The Cority platform is positioned as a user-friendly solution designed by and for quality professionals, focusing on end-to-end management of quality events. Key functional areas highlighted include the ability to systematically address non-conformances, conduct thorough investigations to identify root causes, implement corrective actions (CAPA), and issue controlled change management. By digitizing these processes, the system directly contributes to the bottom line by significantly cutting costs associated with rework, scrap, and expensive product recalls. This focus on operational efficiency and cost reduction, driven by improved quality control, is a primary value proposition. A significant component of the system is the Document Control module, which centralizes the distribution, approval, and storage of all critical quality documents. This centralization is crucial for regulatory adherence, as it enables organizations to quickly and easily retrieve necessary documentation when an audit occurs, thereby streamlining the compliance process. Furthermore, the platform extends its visibility across the entire value chain through dedicated audit and supplier management tools. By consolidating data from these various sources, Cority Analytics proactively identifies risks, trends, and systemic root causes. This proactive approach allows companies to verify product quality *before* it reaches the consumer, protecting both public safety and the brand’s reputation. Finally, the video stresses the technical advantages of the Cority solution, specifically noting its architecture as a true Software-as-a-Service (SaaS) platform. This architecture ensures that updates are delivered smoothly and consistently, guaranteeing that the system always maintains the latest functionality and, critically, remains current with evolving compliance requirements. The overall message encourages regulated companies to abandon inefficient paper processes and adopt modern, automated Quality Management systems to revolutionize their compliance and operational efficiency. Key Takeaways: • **Digital Transformation of Quality Management (QM) is Critical:** Relying on paper-based QM processes results in significant business risks, including lost documents, siloed information, and increased difficulty for Quality Managers, necessitating a shift to automated platforms to meet the speed and complexity demands of modern supply chains. • **Comprehensive Quality Event Lifecycle Management:** Effective QM software must provide tools to manage the entire quality event lifecycle, specifically addressing non-conformances, facilitating root cause investigations, implementing corrective and preventive actions (CAPA), and managing organizational change safely and compliantly. • **Direct Financial Impact of Automation:** Automating quality processes directly impacts profitability by cutting costs associated with operational failures, including reducing rework, minimizing scrap waste, and preventing costly product recalls that damage brand reputation. • **Centralized Document Control for Audit Readiness:** A robust Document Control module is essential for regulatory compliance, ensuring that all quality documents are distributed, approved, and securely stored in one location, allowing for quick and easy retrieval during internal or external audits. • **Proactive Risk Identification through Analytics:** Modern QM platforms must leverage consolidated data across the value chain (including supplier and audit data) to power analytics that proactively identify risks, emerging trends, and systemic root causes, enabling verification of product quality before consumer exposure. • **Visibility Across the Value Chain:** Effective enterprise quality management requires tools for both audit management and supplier management, providing greater visibility and insights into quality performance and compliance adherence throughout the extended supply chain. • **Importance of True SaaS Architecture for Compliance:** Utilizing a true SaaS platform ensures that the quality management system receives smooth, consistent updates, guaranteeing that the software always incorporates the latest regulatory compliance requirements and necessary functionality. • **Protecting Brand Reputation and Consumer Safety:** The ultimate goal of automated quality management is to verify product quality before distribution, which serves the dual purpose of protecting the end consumer from harm and safeguarding the company's brand reputation from the fallout of quality failures. Tools/Resources Mentioned: * Cority’s Enterprise Quality Management (EHSQ) Platform * Cority Analytics * Cority Document Control module Key Concepts: * **Quality Management (QM):** The formal system used to document processes, procedures, and responsibilities for achieving quality policies and objectives, particularly critical in highly regulated sectors like life sciences. * **Non-Conformances:** Instances where a process, product, or service fails to meet specified requirements or standards. The platform is designed to systematically address and resolve these failures. * **Corrective Actions (CAPA):** Actions taken to eliminate the cause of a detected non-conformity or other undesirable situation to prevent recurrence. * **Change Management:** A formalized process used to manage and control changes to organizational processes, systems, or documents to ensure safety, quality, and compliance are maintained during transitions. * **Document Control:** The process of ensuring that all documents related to quality and operations are properly created, reviewed, approved, distributed, and archived, which is a foundational requirement of GxP and 21 CFR Part 11 regulations.

Janssen, Syneos Health, IACT Health: Collaboration in Clinical Trials
Veeva Systems Inc
@VeevaSystems
Feb 1, 2019
This video provides an in-depth exploration of the critical collaboration challenges facing the clinical trial ecosystem, featuring perspectives from major industry players like Janssen, Syneos Health, and IACT Health, and hosted by Veeva Systems. The central theme articulated by the speaker is the pervasive and destructive "us versus them" mentality that hinders efficiency and progress across the life sciences sector. This adversarial mindset is not limited to the relationship between sponsors and their external partners (Contract Research Organizations or CROs, and clinical sites) but often exists internally within large pharmaceutical organizations themselves. The speaker highlights that the current operational environment is characterized by a lack of a shared vision and a failure to prioritize mutual success. Stakeholders operate from a position of individual need, constantly stating "I need more questions to evaluate this patient clinically," or "I need more technologies because this is what I think is going to suit my needs the best." This self-centered approach supplants the necessary collective focus on "we need." Consequently, sponsors fail to seek understanding regarding what sites require to be most effective, what CRO partners need to execute efficiently, and fundamentally, what patients need to ensure successful trial participation. A significant portion of the analysis focuses on the consequences of this siloed thinking. When sponsors complain that CRO partners "just don't understand," the speaker challenges them to consider what proactive steps were taken to provide that necessary vision or context. Similarly, when sites are perceived as failing to prioritize a sponsor’s clinical trials, the speaker suggests that the fault lies with the sponsor for not making the trial easier—through simpler contracts, streamlined budgets, or reduced administrative burden—to encourage site engagement. The ultimate solution proposed is the adoption of a common vision driven by the patient, which should serve as the unifying force for all parties. However, the speaker concludes that the industry currently lacks the "great tools" necessary to bridge these organizational divides, overcome individual points of view, and foster true collaborative success. Key Takeaways: * **Systemic Collaboration Failure:** The clinical trial environment is plagued by a deep-seated "us versus them" mentality, which exists between sponsors, CROs, and sites, and even within different departments of a single large pharmaceutical company. * **Prioritization of Individual Needs:** Stakeholders, particularly sponsors, tend to focus on their own requirements ("I need, I need, I need"—e.g., more data, specific technologies) rather than adopting a collective "we need" perspective centered on partner success. * **Lack of Shared Vision for CROs:** Sponsors frequently lament that their CRO partners "just don't understand" the trial objectives or context, yet they often fail to actively provide the necessary vision, training, or integrated systems required for effective partnership. * **Site Engagement Barriers:** Clinical sites often fail to prioritize specific trials because sponsors have not made the process easy; this includes overly complex contracts, cumbersome budgets, and unnecessary administrative complexity that hinders rapid activation and patient enrollment. * **Patient-Centric Unification:** The patient should serve as the singular, common vision that drives all stakeholders—sponsors, CROs, and sites—to align their efforts and overcome organizational friction. * **Need for Operational Simplification:** To improve site engagement and trial velocity, sponsors must actively simplify their processes, including making contracts simpler, budgets easier to approve, and overall trial execution less burdensome for site staff. * **Technology Gap in Collaboration:** The industry currently lacks robust, integrated tools capable of bridging the communication and operational divides between disparate organizations, preventing the establishment of a single, unified workflow. * **Sponsor Accountability for Partner Success:** Sponsors must shift their mindset from demanding compliance to enabling success, recognizing that the effectiveness of CROs and sites is a direct reflection of the support, clarity, and ease of execution provided by the sponsor. * **Internal Silos Hinder External Collaboration:** The "us versus them" dynamic starts internally within large pharma organizations, suggesting that internal process optimization and data sharing are prerequisites for successful external partnerships. Key Concepts: * **Us Versus Them Mentality:** A pervasive siloed approach in clinical research where different stakeholders (e.g., Sponsor vs. CRO, Sponsor vs. Site) view each other as adversaries or separate entities with conflicting goals, rather than partners working toward a common objective. * **Common Vision:** The concept that all parties involved in a clinical trial—driven by the ultimate goal of patient benefit and successful research—should align their individual objectives and processes under a single, shared purpose.

AbbVie Ventures
AbbVie
/@AbbVie
Jan 7, 2019
This video provides an in-depth exploration of AbbVie Ventures, detailing the pharmaceutical giant's strategic approach to utilizing venture investing as a mechanism to access and integrate promising innovation originating outside of its internal research and development laboratories. The core motivation behind this strategy is the recognition that groundbreaking science is often unproven in its early stages, and external partnerships are essential to translate these nascent innovations into viable new drugs for patients. AbbVie's venture strategy emphasizes active engagement and collaboration rather than passive investment. The firm facilitates deep, continued interactions between its internal R&D teams and those of its portfolio companies. A key example cited is the collaboration between Morphic R&D and the AbbVie R&D team, where ongoing conversations were facilitated as programs required them. This process is crucial for developing strong relationships, building core familiarity with the partner company's technology, and ensuring alignment on scientific goals and development pathways. This hands-on approach is designed to mitigate the risks associated with early-stage science by providing necessary support and integrating the external innovation into AbbVie’s established development pipeline. The video highlights investments in highly ambitious and technologically advanced areas that represent significant leaps in therapeutic potential. Specific portfolio companies mentioned include Rebel Metrics, whose technology holds the potential to open up an entirely new class of biomolecules to be drugged with small molecules—a feat historically deemed impossible. Another example is PACT Pharma, which is pursuing a highly personalized approach to cancer treatment using adoptive cell therapy. These investments underscore AbbVie’s focus on high-risk, high-reward technologies that could fundamentally change therapeutic modalities and require significant data infrastructure to manage. Ultimately, AbbVie Ventures positions itself as a strategic partner, working hand-in-hand with portfolio companies, their management teams, and co-investors. The goal is to provide the guidance and resources necessary for these early-stage companies to succeed and accelerate their scientific programs. The underlying philosophy is driven by the rapid pace of scientific advancement, necessitating that AbbVie’s corporate strategies evolve just as quickly to maintain a competitive edge and ensure timely access to the next generation of therapeutic breakthroughs. ### Detailed Key Takeaways * **Venture Investing as Strategic R&D Sourcing:** AbbVie explicitly uses venture investment as a primary mechanism to gain access to cutting-edge, external innovation, recognizing that the most promising scientific breakthroughs often originate outside the company's internal research labs. * **Acceptance of Early-Stage Risk:** The investment strategy targets early-stage companies where the science is often unproven, demonstrating a willingness to accept inherent scientific risk in pursuit of innovations with the potential to translate into transformative new drugs for patients. * **Mandatory Cross-Functional R&D Integration:** Investment requires active facilitation of communication and collaboration between the portfolio company's R&D teams (e.g., Morphic) and AbbVie’s internal R&D groups to ensure programs receive necessary scientific and logistical support. * **Relationship and Familiarity Building:** Continued, facilitated interactions are essential for developing strong, trust-based relationships and building a core familiarity with the partner company’s specific technology, which is critical for successful long-term integration and eventual commercialization. * **Targeting Foundational Platform Technologies:** Investments are focused on technologies with the potential to "open up an entirely new class" of therapeutic possibilities, such as Rebel Metrics' approach to making previously undruggable biomolecules accessible via small molecules. * **Strategic Focus on Personalized Medicine:** The investment in PACT Pharma, centered on personalized adoptive cell therapy for cancer, signals a strong strategic interest in highly complex, data-intensive, and individualized treatment modalities, which require sophisticated clinical and data management systems. * **Active Partnership and Guidance:** AbbVie works closely with the management teams and co-investors of portfolio companies to provide necessary guidance, positioning itself as an active strategic partner rather than just a passive financial backer. * **Need for Strategic Agility:** The rapid acceleration of scientific discovery necessitates that corporate strategies, particularly those related to R&D sourcing and investment, must move just as quickly to capture emerging opportunities before competitors. * **Data Integration Challenges:** The integration of external, cutting-edge technologies (like personalized cell therapy or novel molecule platforms) requires robust data engineering and integration capabilities to handle diverse, complex, and potentially non-standardized data streams from partner companies. * **Opportunity for AI in R&D Collaboration:** Early-stage scientific collaboration and complex R&D programs are prime areas for deploying specialized AI agents and LLMs to accelerate research, manage complex scientific literature, and facilitate efficient cross-team communication and data synthesis between AbbVie and its portfolio companies. ### Examples/Case Studies * **Morphic R&D Collaboration:** An example of active partnership where AbbVie facilitated conversations between the Morphic R&D team and the AbbVie R&D team as the program’s scientific needs dictated, enabling a strong relationship and familiarity with the technology. * **Rebel Metrics:** A portfolio company focused on developing technology that enables small molecules to drug an entirely new class of biomolecules, which represents a significant technological challenge and opportunity in drug discovery. * **PACT Pharma:** A portfolio company taking an ambitious approach to treating cancer through truly personalized adoptive cell therapy, highlighting AbbVie’s interest in complex, individualized treatment modalities.

RIMs "By the Numbers" A Quick Look Guide to Inproving Your Global Submissions
MasterControl
/@MasterControlVideo
Nov 16, 2018
This video provides an in-depth exploration of Regulatory Information Management (RIMs) and strategies for improving global submissions in the life sciences and medical device industries. Alex Butler, a Product Marketing Manager at MasterControl with extensive experience in product development and regulatory submissions, highlights the increasing complexity of navigating international markets and evolving regulations. The presentation emphasizes that timely device registration is crucial for market success and delves into common challenges faced by regulatory professionals, offering strategic approaches to overcome them. The core of the discussion identifies prevalent issues that impede efficient global submissions. These include the continued reliance on paper-based systems, disparate processes across different teams or regions, inadequate access and control over critical documents, and poor communication channels. Butler presents startling statistics, noting that 70-90% of 510k and PMA submissions to the FDA are rejected at the first review due to clerical and formatting issues, underscoring a systemic problem of overburdened and understaffed regulatory teams. He describes the current regulatory environment as a "perfect storm" due to significant upcoming changes in regions like Canada and Europe (e.g., EU MDR), which demand immediate strategic adaptation. To address these challenges, the presentation advocates for a holistic, global approach to regulatory success. Key strategies include formalizing procedures and processes across the entire organization, ensuring harmonization of practices with international partners, and establishing a "single source of truth" for all regulatory content. This involves robust metadata management, effective dossier management (treating a body of documents as a single record), and seamless integration with other enterprise solutions like ERP, CRM, and Quality Management Systems (QMS). Butler stresses the importance of project management transparency to accurately track regulatory initiatives, prioritize efforts, and communicate the true cost of delays to executives, who often have unrealistic expectations regarding market clearance timelines. A critical insight shared is the need for early and continuous engagement of regulatory teams throughout the product lifecycle. Regulatory professionals should not be siloed or brought in only at the submission phase; rather, they must participate from the initial concept and research (lab book) stages through design history file development and post-market surveillance. This proactive involvement ensures that content is created with regulatory requirements in mind, reducing downstream rejections and delays. The presentation concludes by urging organizations to educate executives on the complexities and burdens faced by regulatory departments, emphasizing that regulatory success is an enterprise-wide responsibility that requires robust tools, formalized processes, and cross-functional collaboration. Key Takeaways: * **Global Regulatory Complexity:** The regulatory landscape is increasingly complex due to evolving international markets and regulations, making global submissions a daunting but critical task for life sciences companies. Changes in regions like Canada and Europe (e.g., EU MDR) are creating a "perfect storm" that demands proactive strategies. * **Common Submission Pain Points:** Regulatory professionals frequently encounter issues such as paper-based systems, disparate processes across teams, lack of controlled access to documents, and poor communication, all of which contribute to significant delays and rejections. * **High FDA Rejection Rates:** A staggering 70-90% of 510k and PMA submissions to the FDA are rejected at the first review, primarily due to clerical and formatting errors, indicating a fundamental breakdown in submission preparation and quality. * **Underestimated Costs of Delays:** Executive expectations for product clearance timelines (e.g., 90 days for 510k) often do not align with reality. Delays in market clearance lead to substantial financial losses and missed market share opportunities, which are often not adequately captured or reported. * **Regulatory Team Understaffing:** The industry's regulatory teams are generally understaffed, with regulatory staffing often around 1% of the organization, leading to overburdened individuals managing three to five complex projects simultaneously, making errors and slippages almost inevitable. * **Holistic Global Submissions Approach:** Companies need to adopt a global strategy that includes formalized internal procedures, harmonized practices with external partners (affiliates, distributors), and robust document and change control mechanisms across all markets. * **Single Source of Truth:** Establishing a centralized, digital "single source of truth" for all regulatory information is paramount. This includes effective metadata management, dossier management (treating a body of documents as a single record), and secure access controls. * **QMS as a RIMs Foundation:** Organizations should leverage and optimize their existing Quality Management Systems (QMS) to function as a Regulatory Information Management (RIMs) solution, rather than investing in separate, disparate systems. This requires thoughtful configuration and integration. * **Project Management Transparency:** Formalizing project management for regulatory initiatives is crucial. This enables clear communication of project status, bottlenecks, and potential slippages to all stakeholders, including executives, and helps prioritize competing initiatives based on impact and resources. * **Early Regulatory Engagement:** Regulatory teams must be engaged much earlier in the product development lifecycle, ideally from the concept and research (lab book) stages, rather than just at the design history file or submission phase. This proactive involvement helps guide testing, design, and content creation to meet regulatory requirements from the outset. * **Regulatory's Unique Role:** Regulatory professionals have a unique and challenging role, often reporting slippages to executives while not being the authors of 90% of the content required for submissions. This necessitates strong cross-functional communication and collaboration. * **Executive Education is Key:** Executives often lack a full understanding of the burdens and complexities faced by regulatory departments. Educating leadership on regulatory needs, timelines, and the impact of non-compliance is essential for securing necessary resources and support. * **Adaptation to Change:** The constant flux in global regulations requires organizations to continuously monitor changes (e.g., EU MDR, MDSAP) and adapt their processes and strategies to minimize damage and ensure ongoing compliance. **Key Concepts:** * **Regulatory Information Management (RIMs):** Solutions or systems designed to manage and track all regulatory information, submissions, and activities throughout the product lifecycle. * **Dossier Management:** The process of organizing, managing, and controlling access to a collection of related documents (a dossier) that support a regulatory submission or product registration. * **Quality Management System (QMS):** A formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives, often used in regulated industries. * **Design History File (DHF):** A compilation of records that describes the design history of a finished medical device, as required by FDA 21 CFR Part 820.30. * **Lab Book:** Refers to the early-stage research and development documentation, often containing proprietary information and initial concepts. * **FDA Refusal to Accept Policy:** An FDA policy outlining the criteria for refusing to accept a submission for review if it is incomplete or contains clerical/formatting errors. * **EU Medical Device Regulation (EU MDR):** A comprehensive regulation governing the production and distribution of medical devices in the European Union, replacing the Medical Device Directive (MDD). * **Medical Device Single Audit Program (MDSAP):** An international program allowing a single audit of a medical device manufacturer's quality management system to satisfy the requirements of multiple regulatory authorities. **Tools/Resources Mentioned:** * **Microsoft Project:** A project management software used for planning, tracking, and managing projects. * **Agile:** A project management methodology that emphasizes iterative development, collaboration, and flexibility. * **MasterControl:** The company hosting the webinar, providing cloud-based quality and compliance software for life sciences and other regulated industries. **Examples/Case Studies:** * **FDA Submission Statistics:** 70-90% of 510k and PMA submissions are rejected at first review due to clerical/formatting issues (from MD UFA quarterly report, Sept 2018). * **Cost of Slippage:** Data from the America Group and LNS reports illustrate the significant financial impact and loss of market share over 10 years for products that are not first or second to market. * **Regulatory Staffing:** Industry data suggests regulatory staffing is often around 1% of an organization, while 25% might be needed for new product development alone, highlighting a severe understaffing issue. * **Customer Examples:** Two customer examples were shared regarding QMS configuration for RIMs: one large company needed less than 20 configuration changes, while a mid-level company needed over 300, emphasizing the importance of a "less is more" approach to metadata.

2018-09-10 TMF Reference Model General Meeting
TMF Reference Model
/@TMFReferenceModel
Nov 14, 2018
This video provides an in-depth exploration of the TMF Reference Model, focusing on community updates, subgroup progress, and the release of version 3.1.0. The general meeting, held in September 2018, served as a platform for various working groups to present their advancements and solicit community involvement, highlighting the collaborative effort behind maintaining and evolving this critical standard for clinical trial documentation. The discussion underscores the model's role in standardizing Trial Master Files (TMFs) across the pharmaceutical and life sciences industries, addressing challenges related to document management, regulatory compliance, and digital information exchange. The meeting progressed through several key updates, beginning with an overview of the TMF Reference Model community's growth, including new project team members, subscribers, and forum participants. Following logistical announcements, the core of the meeting involved detailed reports from specialized subgroups. These included updates on the Non-Interventional Study (NIS) TMF, which is being segmented into three categories for different study types; the Sub-Artifacts team, which is diligently defining sub-artifacts across various TMF zones and seeking additional reviewers; and the Device TMF group, which is reviewing zones to develop a high-level best practices guide and suggesting modifications to artifact names and commentary. A significant portion of the meeting was dedicated to the "Framework for the Destruction of Paper," detailing its project timeline, parameter review, and the development of implementation tools such as decision trees and policy templates, emphasizing the ongoing need for volunteers. A crucial segment of the meeting focused on the Exchange Mechanism Standard (EMS) team, which had recently hosted a business-focused webinar and planned future technical and business webinars to encourage piloting of the EMS by CROs and sponsors. The highlight of the meeting was the announcement of the TMF Reference Model version 3.1.0 release by the Change Control Board (CCB). This "minor update," effective October 10th, incorporated 23 approved change requests out of 64 submitted since 2015. Key changes included the integration of dating conventions and milestones (country, trial, site levels) directly into the model, minor artifact name adjustments, updated definitions, the addition of a few sub-artifacts, correction of typographical errors, and updates to filing levels and alternate names. The meeting concluded with a discussion on the impact of this new version, emphasizing that while changes were minimal and primarily aimed at consistency, the stable artifact ID numbers ensure seamless data exchange and mapping, even with evolving artifact names. Key Takeaways: * **TMF Reference Model as a Core Standard:** The TMF Reference Model is a foundational standard for managing clinical trial documentation, crucial for regulatory compliance and operational efficiency within the pharmaceutical and life sciences sectors. Its continuous evolution reflects industry needs and best practices. * **Community-Driven Development:** The model's development and updates are a collaborative effort, relying on a large community of volunteers, project teams, and subgroups. This ensures broad industry representation and practical relevance. * **Version 3.1.0 Release Details:** The TMF Reference Model version 3.1.0 is a minor update, incorporating 23 approved change requests. Key enhancements include integrated dating conventions, country/trial/site level milestones, minor artifact name adjustments, and refined definitions to improve clarity and consistency. * **Stable Artifact IDs for Data Exchange:** Despite changes to artifact names or definitions, the underlying artifact ID numbers remain constant. This is critical for data mapping, migration, and the interoperability of TMF systems, particularly when using the Exchange Mechanism Standard (EMS). * **Importance of Detailed Release Notes:** Users are strongly encouraged to consult the detailed release notes for version 3.1.0, which provide comprehensive information on all changes, including old and new text, to understand the full scope of updates. * **Subgroup Progress and Future Outlook:** Active subgroups are addressing specific areas such as Non-Interventional Studies (NIS), Device TMFs, and the comprehensive definition of sub-artifacts. These efforts are expected to lead to future major releases incorporating new frameworks and guidelines. * **Framework for Paper Destruction:** A dedicated framework is being developed to guide the compliant destruction of paper TMF documents. This project involves creating tools like decision trees and policy templates, highlighting the industry's move towards digital solutions while managing legacy paper records. * **Exchange Mechanism Standard (EMS) Adoption:** The EMS team is actively promoting the standard through webinars and seeking sponsors, CROs, and vendors to pilot its implementation. This initiative aims to standardize the digital exchange of TMF content between different organizations and systems. * **Call for Volunteers:** The TMF Reference Model initiative consistently seeks volunteers for various roles, including reviewing sub-artifacts, participating in zone teams, and assisting with implementation projects like the paper destruction framework. This collaborative model is essential for its ongoing success. * **Impact of Minor Updates:** Minor updates like version 3.1.0 are unlikely to cause significant incompatibility issues with previous versions. Organizations do not necessarily need to immediately update their systems but can incorporate changes during planned TMF structure reviews. * **Feedback and Change Request Process:** The community is encouraged to submit feedback and change requests through a dedicated online forum, providing specific details (e.g., artifact numbers) to facilitate review by the Change Control Board. The forum is not for general questions, which should be directed to community groups. * **Consideration for Artifact-Level Versioning:** A discussion point raised the potential for versioning at the individual artifact level, rather than just the entire reference model. While challenging for non-technical users or paper-based systems, this could be beneficial for eTMF systems capable of tracking granular changes. Tools/Resources Mentioned: * MailChimp (for mailing lists/newsletters) * Yahoo Groups (for online discussions) * Groups.IO (for project team members) * TMF Reference Model Website (for general information, meeting details, and downloading ICS files) * Outlook/Google Calendar (for importing meeting details via ICS files) * Online Forum (for submitting feedback and change requests) Key Concepts: * **TMF Reference Model:** A standardized, hierarchical model for organizing and managing documents within a Trial Master File, ensuring consistency and regulatory compliance. * **Artifacts:** Individual documents or records within the TMF, each assigned a unique ID number. * **Sub-Artifacts:** More granular components or types of documents within a broader artifact category. * **Zones:** High-level categories within the TMF Reference Model (e.g., Study Management, Central Trial Documents). * **Change Control Board (CCB):** The governing body responsible for reviewing, approving, and managing changes to the TMF Reference Model. * **Exchange Mechanism Standard (EMS):** A standard designed to facilitate the digital exchange and interoperability of TMF content between different systems and organizations. * **Non-Interventional Studies (NIS):** Clinical studies where patients receive routine care, and data is collected without intervention, requiring specific TMF considerations. * **Device TMF:** The Trial Master File specific to medical device studies, which may have unique documentation requirements compared to pharmaceutical trials. * **Framework for Destruction of Paper:** A guideline or methodology for the compliant and systematic destruction of physical TMF documents, particularly relevant as organizations transition to electronic TMFs. * **Version Control (Maintenance, Minor, Major Changes):** A classification system used by the CCB to categorize the impact and scope of updates to the TMF Reference Model.
Pull Approved Content from Veeva Vault Promomats into Entatio.com
MediaManager
/@mediamanager465
Nov 14, 2018
This video provides an in-depth exploration of integrating Veeva Vault PromoMats with Entatio.com to streamline the distribution of regulatory-approved content for life sciences companies. The presenter begins by establishing the critical need for pharmaceutical and biotech firms to share promotional and educational materials, such as prescriber information and sales presentations, with healthcare providers while rigorously adhering to regulatory compliance. The core problem addressed is how to efficiently make content that has undergone Promotional Review Committee (PRC) approval available to an audience, ensuring that only approved and current versions are distributed. The tutorial then walks through the practical steps of connecting a Veeva Vault instance to Entatio.com. It highlights Veeva Vault's role as a popular regulatory document management system that facilitates the PRC review process for marketing content. By integrating these two platforms, Entatio ensures that any content shared with the healthcare community has been officially reviewed and approved for release. The demonstration includes navigating the Entatio interface to set up vault credentials, browse available content within the vault, and select specific media, such as an "interactive visual aid" (IVA) or "closed-loop marketing presentation," for import. A significant aspect of the integration is the capability for automated content synchronization. The video details how users can configure Entatio to automatically check for updates to the selected media in Veeva Vault on a scheduled basis (e.g., daily or weekly). This feature ensures that if any changes are approved in Veeva Vault, the distributed content in Entatio is automatically updated, and subscribers (such as healthcare providers) can be notified via email. The presentation concludes by illustrating how the content appears to the end-user and emphasizes the crucial integration with Veeva CRM, which allows life sciences companies to track specific individuals' engagement with the distributed media, thereby providing valuable insights for commercial operations. Key Takeaways: * **Ensuring Regulatory Compliance in Content Distribution:** The video underscores the paramount importance of distributing only Promotional Review Committee (PRC) approved content, especially for life sciences companies sharing sensitive information like prescriber data or sales presentations with healthcare providers. Veeva Vault PromoMats serves as the central hub for this regulatory approval process. * **Streamlined Content Integration from Veeva Vault:** Entatio.com offers a direct and user-friendly method to connect to Veeva Vault, enabling life sciences companies to pull approved media assets seamlessly. This integration simplifies the process of making compliant content available to a wider audience. * **Automated Content Synchronization for Accuracy:** A key feature demonstrated is the ability to schedule automatic updates, where Entatio periodically checks Veeva Vault for any changes to linked media. This ensures that the content presented to the audience is always the most current, PRC-approved version, minimizing the risk of distributing outdated or non-compliant materials. * **Enhanced Content Management and Organization:** Within Entatio, users can create "presentations" or collections of media, allowing for structured organization of content pulled from Veeva Vault. This facilitates easier management and distribution of various interactive visual aids (IVAs) or closed-loop marketing (CLM) presentations. * **Audience Subscription and Notification System:** The platform supports a subscription model where healthcare providers or other audience members can subscribe to specific content. Upon any update to the content in Veeva Vault (and subsequent automatic synchronization to Entatio), subscribers receive automated email notifications, keeping them informed of new information. * **Robust Engagement Tracking with Veeva CRM:** The integration extends to Veeva CRM, allowing life sciences companies to track individual user engagement with the distributed media. This provides critical data for sales and marketing teams, offering insights into which healthcare professionals are viewing specific content, thereby enhancing commercial operations and sales effectiveness. * **Flexible Content Embedding Options:** Approved content can be embedded into external websites, offering flexibility in how it is shared. This can be done with or without a registration screen, catering to different access control requirements and marketing strategies. * **Reference Numbering for Traceability:** When content is pulled from Veeva Vault, Entatio retains the original vault reference number. This ensures clear traceability back to the source document in the regulated system, which is crucial for audit trails and compliance verification. * **Optimized Content Marketing and Sharing:** By combining a regulatory document management system (Veeva Vault) with a content distribution platform (Entatio) and a CRM system (Veeva CRM), life sciences companies can achieve a more efficient, compliant, and insightful content marketing and sharing ecosystem. Tools/Resources Mentioned: * **Veeva Vault PromoMats:** A regulatory document management system widely used in the pharmaceutical and life sciences industries for managing and approving promotional and marketing content. * **Entatio.com:** A content distribution platform demonstrated in the video for sharing and tracking approved media with audiences, particularly within the healthcare community. * **Veeva CRM:** A customer relationship management platform, specifically mentioned for its integration with Entatio to track user engagement with distributed content. Key Concepts: * **Promotional Review Committee (PRC):** A critical internal process in life sciences companies where marketing and promotional materials are reviewed by medical, legal, and regulatory teams to ensure compliance with industry regulations and internal policies before public release. * **Interactive Visual Aid (IVA) / Closed-Loop Marketing (CLM) Presentation:** Digital sales tools used by pharmaceutical sales representatives during interactions with healthcare professionals. These are often interactive and designed to capture feedback or data, forming a "closed loop" of information. * **PII (Prescriber Information):** Personally Identifiable Information related to prescribers (healthcare professionals), which requires careful handling and compliance with data privacy regulations. * **Auto-Update Feature:** A mechanism that automatically synchronizes content from a source system (like Veeva Vault) to a distribution platform (like Entatio) whenever changes are made and approved in the source, ensuring content remains current.
Logging into ETQ Academy
ETQ Reliance: Leading Quality Management System
/@ETQReliance
Nov 7, 2018
This video serves as a concise, procedural guide for new users accessing ETQ Academy, the dedicated learning management system (LMS) for ETQ Reliance, a leading Quality, EHS, and Compliance management software suite. The primary objective is to ensure users can successfully navigate the initial login and password setup process to begin their mandatory training. The instructional approach is direct and sequential, emphasizing the importance of following specific steps to gain access to critical system knowledge. The process begins with the user locating a welcome email originating from "Academy at etq.com." This email is the gateway to the system, containing the user’s unique login ID and, crucially, a button hyperlink designed to initiate the password creation sequence. Upon clicking this link, the user is routed to the ETQ Academy home page, where they are prompted to set and confirm a new password. This initial security step is foundational for regulated systems, ensuring user identity verification before accessing training materials related to quality and compliance. Once logged in, the video highlights the immediate requirement for all new users to complete the "Getting Started at ETQ Academy" program. This program is presented as the mandatory starting point, structured as a learning path that begins with an "Overview for Learners" before progressing through subsequent courses. The emphasis on a structured learning path underscores the necessity of standardized training in regulated environments, ensuring all users achieve a baseline understanding of the QMS platform's functionality and compliance requirements. The video concludes by stressing that completing this initial program is essential for fully understanding how ETQ Academy operates and how users will manage their ongoing training requirements within the system. Key Takeaways: • **Mandatory Structured Onboarding for QMS:** The video highlights the necessity of a formal, structured "Getting Started" program within ETQ Academy. For pharmaceutical and life sciences clients, this mandatory training ensures that personnel interacting with the Quality Management System (QMS) are properly trained, which is a critical requirement for GxP and 21 CFR Part 11 compliance. • **Importance of Training Documentation:** The use of a dedicated LMS (ETQ Academy) for QMS training ensures that comprehensive audit trails and training records are automatically generated and maintained. This capability is vital for regulatory audits, demonstrating that the workforce is qualified and trained on the validated software (ETQ Reliance). • **ETQ Reliance as a Key Regulated Platform:** The context establishes ETQ Reliance as a major enterprise Quality, EHS, and Compliance management system. For IntuitionLabs.ai, understanding the operational and training infrastructure of this platform is key to offering effective data integration and AI solutions that interact with core quality data. • **Standardized Access Protocol:** The reliance on a specific welcome email and a defined password setting procedure minimizes access errors and ensures security integrity. This disciplined approach to user provisioning is standard practice for regulated enterprise software, which IntuitionLabs must replicate in its custom solutions. • **Focus on User Proficiency:** The entire training structure, starting with the "Overview for Learners," is designed to rapidly bring users to proficiency on the QMS. This focus aligns with commercial operations goals of minimizing downtime and ensuring quality processes are executed correctly from day one. • **Integration Opportunity for AI Tools:** Since ETQ Reliance manages critical compliance data (CAPAs, deviations, audits), the training environment provides context on how users interact with quality data. This insight is valuable for developing AI agents or LLM solutions that can assist sales operations or medical affairs teams by synthesizing or summarizing quality-related information derived from the QMS. • **Training as a Validation Component:** In the life sciences sector, training on the QMS is often considered part of the system validation lifecycle. The existence of a formal training academy confirms that ETQ provides the necessary tools for clients to meet the "training and competence" requirements of regulatory bodies. Tools/Resources Mentioned: * **ETQ Academy:** The Learning Management System (LMS) used for training on the ETQ Reliance platform. * **ETQ Reliance:** The core Quality, EHS, and Compliance management software suite. Key Concepts: * **Quality Management System (QMS):** An integrated system used by organizations to document processes, procedures, and responsibilities for achieving quality policies and objectives, essential for regulatory compliance in life sciences. * **Structured Learning Path:** A predefined sequence of courses or modules designed to ensure comprehensive coverage of necessary topics, particularly crucial for training on regulated software to ensure GxP adherence. * **User Onboarding in Regulated Environments:** The formal process of introducing new users to a validated system, which must include documented training and competency checks to satisfy requirements like 21 CFR Part 11.

Veeva Systems CEO: Modernizing Manufacturing | Mad Money | CNBC
CNBC Television
/@CNBCtelevision
Oct 18, 2018
This video features an interview with Peter Gassner, founder and CEO of Veeva Systems, on CNBC's Mad Money, discussing the company's significant growth and strategic direction within the life sciences industry. Jim Cramer positions Veeva as a leading cloud-based software provider for pharmaceutical, biotech, and life sciences companies, highlighting its role in enhancing pharmaceutical sales effectiveness, capturing clinical trial data, and ensuring compliance with government regulations. The conversation underscores Veeva's impressive financial performance, including tripling products, quadrupling revenues, and sextupling profits over five years, achieving its billion-dollar revenue goal a year ahead of schedule. Gassner elaborates on Veeva's expansion strategy, likening it to the successful models of Salesforce and Adobe, by developing both a "development cloud" and a "commercial cloud" tailored for the life sciences sector. A central theme is the industry's ongoing digital transformation, moving away from outdated paper-based processes that have historically burdened highly regulated fields. He emphasizes that while life sciences is a serious, $1.6 trillion business focused on improving human life, many critical procedures, especially those related to patient safety and regulatory compliance, have remained manual. Veeva's mission is to modernize these processes with cloud software, making operations more efficient and improving job satisfaction for professionals in these companies. The interview also explores Veeva's strategic move to expand its market beyond traditional life sciences. Gassner introduces "Quality One," a product designed to address similar paper-based and client-server burdens in the manufacturing and distribution processes of other regulated industries, such as cosmetics, chemicals, and consumer packaged goods. This expansion signifies a broader recognition of the need for specialized cloud solutions in any sector where careful manufacturing and distribution are critical and subject to stringent oversight, indicating a vast untapped market for Veeva's expertise in regulated enterprise software. Key Takeaways: * **Dominant Cloud Software Provider:** Veeva Systems is a leading cloud-based software company specifically serving the pharmaceutical, biotech, and life sciences industries, demonstrating sustained growth and market leadership. Its offerings span critical functions like commercial operations, clinical trial data management, and regulatory compliance. * **Strategic Expansion and Growth:** Veeva has achieved remarkable growth, tripling its product offerings, quadrupling revenues, and sextupling profits in five years, reaching its billion-dollar revenue target ahead of schedule. This growth is fueled by a strategic expansion into both "development cloud" and "commercial cloud" solutions. * **Digital Transformation in Life Sciences:** The life sciences industry, despite its forward-thinking nature in research, has historically relied heavily on paper-based processes for regulatory compliance and operational procedures, particularly concerning patient safety. This presents a significant opportunity for digital modernization through specialized cloud software. * **Regulatory Compliance as a Driver:** The inherent regulatory complexity of the life sciences sector (e.g., FDA, EMA, GxP) necessitates robust, compliant software solutions. Veeva's success is partly attributed to its ability to help clients navigate and comply with these stringent government regulations, moving away from inefficient manual systems. * **Replicating Successful SaaS Models:** Veeva's strategy mirrors the expansion models of tech giants like Salesforce and Adobe, by continuously innovating and expanding its product portfolio within its niche, thereby deepening customer relationships and increasing its total addressable market. * **Modernizing Manufacturing Beyond Pharma:** Veeva is extending its expertise beyond core life sciences with its "Quality One" product, targeting manufacturing and distribution in other regulated industries such as cosmetics, chemicals, and consumer packaged goods. This indicates a broader market need for cloud-based solutions to replace outdated paper and client-server systems in quality-critical environments. * **Impact on Employee Efficiency and Satisfaction:** Modern cloud software not only enhances operational efficiency and compliance but also improves the daily work experience for professionals in life sciences, allowing them to focus more on their core mission of making medicines and less on administrative burdens. * **Vast Untapped Market for Regulated Software:** The discussion highlights a significant market opportunity in providing specialized, compliant cloud software for any industry with complex, regulated manufacturing and distribution processes that are currently hampered by legacy systems. * **Importance of Industry-Specific Solutions:** The success of Veeva underscores the value of deeply understanding a specific industry's unique challenges, regulatory landscape, and operational needs to develop tailored software solutions that generic platforms cannot adequately address. Tools/Resources Mentioned: * **Veeva CRM:** A leading platform for pharmaceutical commercial operations. * **Veeva Vault:** A suite of cloud applications for content and data management across R&D, clinical, quality, and commercial operations. * **Quality One:** A new Veeva product aimed at modernizing quality and manufacturing processes in regulated industries beyond life sciences (e.g., cosmetics, chemicals, CPG). * **AWS (Amazon Web Services):** Mentioned as a common cloud migration target, implying Veeva's own cloud infrastructure is robust and modern. Key Concepts: * **Commercial Cloud:** Software solutions focused on sales, marketing, and customer relationship management within an industry. * **Development Cloud:** Software solutions supporting research and development, particularly in areas like clinical trials and regulatory submissions. * **Digital Transformation:** The process of adopting digital technology to fundamentally change how an organization operates and delivers value. * **Regulatory Compliance:** Adherence to laws, regulations, guidelines, and specifications relevant to a particular industry, crucial in life sciences for patient safety and product efficacy.

VISEVEN | 2018 | Webinar | Evolve your expertise in Veeva
Viseven
/@VisevenMarTech
Oct 2, 2018
This webinar, presented by Viseven, focuses on evolving Veeva expertise, particularly in managing multichannel content for pharmaceutical and life sciences companies. The speaker introduces e-Wizard, a unique platform designed to simplify content creation, localization, adaptation, and publishing to various Veeva solutions such as Veeva CRM, Veeva Vault PromoMats, Veeva Approved Email, Veeva CLM, Veeva CoBrowse, and Veeva MyInsights. The discussion highlights how e-Wizard addresses common challenges like the need for specialized knowledge, human resources, and high costs associated with traditional content management, offering a solution that streamlines workflows, ensures compliance, and provides actionable insights for commercial operations. Key Takeaways: * **Veeva Ecosystem Integration:** The e-Wizard platform offers deep, two-way integration with a comprehensive suite of Veeva solutions (CRM, Vault PromoMats, Approved Email, CLM, CoBrowse, MyInsights), enabling seamless content management and data synchronization across the pharmaceutical commercial ecosystem. * **Content Lifecycle Optimization:** e-Wizard drastically simplifies and accelerates the content lifecycle, from creation and localization to approval and publishing, by providing tools for converting various file types to HTML5 CLM, managing a global store of approved content, and offering a component-based templating approach for consistent branding and efficient adaptation. * **Enhanced Commercial Operations & Analytics:** The platform supports the creation of interactive presentations, remote visits, and customizable dashboards (leveraging Veeva MyInsights) to deliver targeted interactions, track detailed KPIs (e.g., slide duration, prescriptions, email open rates), and provide rich analytics for real-time monitoring of communication channel efficiency. * **Reduced Operational Overhead:** By automating content publishing and providing flexible tools, e-Wizard aims to reduce reliance on external agencies, minimize administrative work, and significantly cut down the time and cost associated with managing and deploying compliant marketing and sales content within the regulated life sciences industry. * **Compliance and Reusability:** The platform emphasizes the use of approved content, master templates, and a component library to ensure global branding consistency, facilitate effortless localization, and maintain regulatory compliance while maximizing content reusability across different channels and regions.
A Day in the Life of a TMF Document Overview
LMK Clinical Research Consulting
/@lmkclinicalresearchconsult7189
Sep 27, 2018
This video provides an in-depth exploration of the Trial Master File (TMF) document lifecycle, emphasizing its critical role in clinical studies and inspection readiness. Presented by Jack Morell, Director of Clinical Operations at LMK Clinical Research Consulting, the webinar systematically breaks down the TMF journey from infrastructure and document creation to ongoing quality control, metrics, and ultimately, regulatory inspections. Morell argues that the TMF is arguably the most important aspect of a clinical study, serving as the foundation for assessing trial conduct, data integrity, and compliance with Good Clinical Practice (GCP). The presentation begins by establishing the foundational TMF infrastructure, detailing the essential processes and tools required for continuous inspection readiness. This includes formally documented SOPs, work instructions, and job aids, alongside mandatory training for all TMF personnel. Key expectations for TMF timeliness, quality, and completeness are defined, with a strong recommendation for using TMF indices—such as the TMF Reference Model or Oasis Reference Model—to outline all expected documentation beyond the basic ICH GCP Section 8 list. The discussion then moves into the document's lifecycle, from its creation and initial QC by the submitter to its proper indexing and submission into the TMF, highlighting specific quality criteria for both paper and electronic documents, including considerations for hyperlinks and image quality for scanned files. A significant portion of the webinar is dedicated to TMF Quality Control (QC), distinguishing between a mere inventory and a true, comprehensive review. Morell stresses the importance of proactive, ongoing QC throughout the study's duration, utilizing tools like storyboards and expected document lists to identify missing documentation. He outlines three types of QC—prospective, retrospective, and oversight—and various timing approaches (milestone, calendar, phase, risk-based). The speaker also addresses the common misuse of "notes to file," advocating for prompt and thorough corrective actions. The final segments delve into the power of TMF metrics for gauging overall TMF health, providing examples of quality metrics broken down by protocol, site, functional line, and QC finding type, and how these can trigger process improvements or retraining. The webinar concludes with a focus on inspection readiness, reiterating that preparation starts on day one and offering practical do's and don'ts for navigating regulatory inspections by agencies like the FDA, MHRA, and EMA, noting how eTMF systems offer inspectors real-time visibility into filing activities. Key Takeaways: * **TMF as the Foundation of Clinical Studies:** The TMF is not merely a collection of documents but the core evidence for assessing clinical trial conduct, data integrity, and GCP compliance, making it paramount for regulatory inspections. * **Robust TMF Infrastructure is Crucial:** Establish formally documented processes (SOPs, work instructions, job aids) and ensure all personnel are trained. This sets the stage for continuous inspection readiness from day one. * **Define Clear Expectations:** TMF processes must clearly define expectations for document timeliness (e.g., submission deadlines), quality (QC criteria, acceptable error rates), and completeness (all expected documentation). * **Utilize TMF Indices:** Go beyond ICH GCP Section 8. Implement a comprehensive TMF index (e.g., TMF Reference Model, Oasis Reference Model) tailored to company SOPs to outline all expected documentation and assign filing responsibilities. * **Quality Starts at Creation:** Every document creator should perform an initial QC before submission to the TMF. This proactive approach minimizes downstream issues and ensures quality standards are met. * **Comprehensive Document QC Criteria:** Beyond basic checks, QC should confirm document appropriateness, finality, completeness (all fields, signatures), legibility, correct page order, and redaction of PII. Special considerations apply to electronic documents (hyperlink functionality, compatible formats, unlocked files) and scanned paper documents (image quality, orientation). * **Continuous TMF QC is Essential:** Implement prospective and oversight QC throughout the study, not just at the end. Use tools like storyboards and expected document lists to identify gaps and ensure ongoing inspection readiness. * **Prompt Corrective Action:** "Find it, fix it" is the motto for QC findings. Resolve issues promptly, ideally within 30 days, as delays can make resolution difficult (e.g., personnel leaving the company). * **Avoid Overuse of "Notes to File":** Notes to file are rarely sufficient for closing QC findings. If used, they must be informative, detailing the issue, attempts to remedy it, and corrective actions to prevent recurrence. * **Leverage TMF Metrics for Health Assessment:** Implement a metrics program (starting simple with quality, completeness, timeliness) to gauge overall TMF health. Metrics can identify systemic issues, prompt process improvements, retraining, or resource allocation changes. * **Inspection Readiness is Day-One Responsibility:** Preparation for inspections begins at the start of the study. Ensure adherence to GCP, understand regulatory environments, follow established SOPs, and have a TMF index and plan in place. * **Beware of Inspection Pitfalls:** Avoid relying solely on ICH GCP, making assumptions about regulations, passing all responsibility to CROs (sponsors are ultimately accountable), or panic-filing documents just before an inspection, as eTMF systems provide inspectors visibility into filing timelines. * **Strategic Inspection Conduct:** During an inspection, only answer questions related to your role, avoid volunteering unrequested information, and do not comment on quality issues or admit non-compliance. Never fabricate documents. Tools/Resources Mentioned: * **TMF Reference Model (DIA Reference Model):** A commonly used industry model for TMF structure. * **Oasis Reference Model:** Mapped structurally to the TMF Reference Model, with added metadata. * **SOPs, Work Instructions, Job Aids:** Formal documentation for TMF processes. * **TMF Indices:** Study-specific roadmaps for expected documentation and filing locations. * **TMF QC Plan:** Defines who, what, and when for QC activities. * **TMF QC Tracker:** Used to document QC findings and their resolution. * **QC Certificate:** Documentation filed in the TMF confirming a QC review was performed. * **Storyboard:** Tracks unique study information, milestones, and events. * **Expected Document List:** Generated at study start and updated to track anticipated documentation. * **eTMF Platforms:** Electronic Trial Master File systems, often configured with reference models, allowing for tracking QC and metrics. * **Excel/SharePoint:** Can be used for TMF QC trackers. Key Concepts: * **TMF (Trial Master File):** A collection of essential documents that individually and collectively permit the reconstruction of the conduct of a clinical trial. * **eTMF (Electronic Trial Master File):** A digital system for managing TMF documents, offering benefits like real-time visibility and access. * **GCP (Good Clinical Practice):** An international ethical and scientific quality standard for designing, conducting, recording, and reporting trials that involve the participation of human subjects. * **ICH GCP Section 8:** A section within the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6(R2) guideline that lists essential documents for the clinical trial. * **Prospective QC:** Ongoing quality control performed regularly throughout the study's duration. * **Retrospective QC:** Quality control performed after a period of non-ongoing QC, generally not recommended. * **Oversight QC:** QC performed by a sponsor on a CRO's TMF, or by a QA group, to ensure adherence to processes. * **Note to File (NTF):** A document used to explain minor deviations or missing information in the TMF, which should be informative and detail corrective actions. Examples/Case Studies: * **Quality Metric Breakdown:** Examples of TMF quality metrics were shown, illustrating how a total document failure rate (e.g., 8% exceeding a 5% threshold) can be broken down by document level, functional line (e.g., country and site management team having a higher error rate), or QC finding type (e.g., high number of duplicate documents indicating unclear filing responsibilities). * **Inspection Readiness Pitfall - Panic Filing:** A graph from Andy Fisher (MHRA inspector) demonstrated a significant spike in document filing immediately before an inspection date, highlighting how eTMF systems reveal such activities to inspectors and can lead to findings.

10 Ways to Revamp Your TMF QC Process
LMK Clinical Research Consulting
/@lmkclinicalresearchconsult7189
Sep 27, 2018
This video provides an in-depth exploration of how to revamp the Trial Master File (TMF) Quality Control (QC) process, emphasizing its critical role in ensuring regulatory compliance and inspection readiness for clinical trials. Julia Devan, President and CEO of LMK Clinical Research Consulting, guides attendees through the "why" and "how" of effective TMF QC, drawing upon her extensive industry experience and LMK's specialized services. The presentation highlights common pitfalls in TMF management, such as delayed filing and inconsistent QC practices, and offers a structured approach to overcome these challenges. The discussion begins by underscoring the importance of TMF QC, citing regulatory expectations from agencies like the MHRA and EMA, which mandate that TMFs be complete and updated in a timely manner, contemporaneous with the clinical trial. Devan illustrates the common problem of documents being filed only at the end of a study or in anticipation of an inspection, stressing the need for a linear distribution of TMF documents throughout the trial. She then delves into the difficulties associated with TMF QC, such as defining clear expectations for document versions, piecing together the study narrative, and navigating centralized versus decentralized QC models, where either a dedicated team or functional lines perform the review. The core of the webinar is a detailed breakdown of ten actionable strategies to enhance TMF QC. These strategies cover foundational elements like developing a comprehensive TMF QC plan, utilizing appropriate tools (e.g., a company-specific TMF Table of Contents mapped to the TMF Reference Model), and ensuring qualified personnel are performing QC. Devan emphasizes the importance of consistent timing for QC activities, standardization of pass/fail criteria, and establishing quality levels with associated risk assessments. A key concept introduced is linking TMF documents to study milestones and events to better define expectations for completeness. The presentation culminates with the necessity of performing a "true review" of the TMF, going beyond a mere inventory check, and implementing prompt, verifiable corrective actions for any identified discrepancies to maintain a constant state of inspection readiness. Key Takeaways: * **TMF QC is Paramount for Compliance:** The Trial Master File (TMF) is the foundation for regulatory inspections, and its quality control is essential for demonstrating patient rights and safety protection, as well as data reliability, aligning with ICH GCP R2 and expectations from agencies like the EMA and MHRA. * **Timeliness is a Regulatory Mandate:** Regulatory bodies expect the TMF to be contemporaneous with the clinical trial, meaning documents should be filed and updated in a timely, ongoing manner, not accumulated and filed en masse at the study's conclusion. * **Address Common QC Challenges:** Many organizations struggle with TMF QC due to difficulties in defining document expectations (e.g., number of versions), piecing together the study's story, and resource constraints, highlighting the need for a structured approach. * **Develop a Comprehensive TMF QC Plan:** A robust QC plan should detail who is responsible, how QC is performed, logistical controls (e.g., expected number of document iterations), timing, and the process for results and corrective actions, and should be system and file structure agnostic. * **Leverage Appropriate Tools:** Utilize a company-specific TMF Table of Contents (mapped from the TMF Reference Model), a list of study SOPs with start/stop dates, and study reference documents (protocol, CTMS data, country lists) to guide the QC process and define expectations. * **Prioritize Qualified and Trained Personnel:** Effective TMF QC requires experienced individuals who understand clinical trials and the specific study, supported by thorough training to ensure consistent review, whether in a centralized or decentralized QC model. * **Establish a Sustainable QC Schedule:** Implement a consistent and manageable QC schedule aligned with company SOPs and document lifecycles, avoiding overburdening staff and ensuring documents are not reviewed redundantly to prevent frustration and non-compliance. * **Standardize Pass/Fail Criteria:** Clearly define what constitutes a "pass" or "fail" for TMF documents based on criteria such as presence, complete signatures, complete fields, and adherence to dating conventions, ensuring consistency across all reviewers. * **Calculate and Track TMF Quality:** Implement a system to calculate TMF QC pass/fail rates for each study, creating a quality score that helps track improvement or decline over time and informs necessary interventions, while also considering the risks associated with missing or poor-quality documents. * **Link Documents to Milestones and Events:** Assign each TMF document to a specific study milestone (e.g., first subject first visit) or event (e.g., protocol amendment) to clearly define when documents are expected to be filed, aiding in completeness checks and timely filing. * **Perform a "True Review," Not Just Inventory:** TMF QC should go beyond a simple inventory check. It requires an in-depth review of documentation against study context, operational plans (e.g., monitoring plan), and regulatory requirements to ensure accuracy, completeness, and proper location. * **Implement Prompt Corrective Actions:** "If you find it, fix it." Discrepancies should be corrected as soon as possible, ideally within two weeks, by following up with responsible parties, verifying corrections, and documenting due diligence if a correction is impossible (e.g., in a monitoring visit report, not a note to file). * **Technology is a "Frenemy":** While eTMF systems offer advanced functionalities, they are only effective when supported by proper processes and trained people. Organizations should ensure their technology works for them, rather than working for the technology. * **Sponsor Oversight is Non-Negotiable:** Under ICH GCP R2, sponsors are ultimately responsible for the TMF, even if delegated to a CRO or FSP. Sponsors must have a TMF QC plan and processes in place to demonstrate effective oversight of their partners. **Tools/Resources Mentioned:** * **TMF Reference Model:** An industry standard for TMF structure and content. * **CTMS (Clinical Trial Management System):** Used to track study progress and provide information relevant to TMF QC. * **Excel Trackers:** Mentioned as a historical or alternative method for managing study information. * **LMK's TMF Complete Service:** A comprehensive TMF QC review process based on ICH GCP R2 and Six Sigma principles. * **LMK's TMF University Program:** An award-winning training program with 18 TMF courses across three learning levels. * **Claire:** An AI chatbot feature on LMK's website for TMF questions. **Key Concepts:** * **TMF QC Plan:** A documented strategy outlining how TMF quality control will be performed, including responsibilities, timing, methods, and corrective actions. * **Centralized vs. Decentralized QC:** Two models for TMF QC: centralized involves a dedicated group (potentially not involved in the study), while decentralized involves functional lines or document owners performing QC on their own documents. * **Milestones and Events (in TMF context):** Specific points in a clinical trial (milestones like first subject first visit) or occurrences (events like a PI change) that trigger the creation or collection of specific TMF documents. * **True Review:** An in-depth assessment of TMF documents that goes beyond a simple inventory check, evaluating content against study context, operational plans, and regulatory requirements. * **Frenemy (Technology):** A term used to describe technology (like eTMF systems) that, while powerful, can become a hindrance if not supported by proper processes and trained personnel. **Examples/Case Studies:** * **Delayed TMF Filing Graph:** A visual representation showing a common pattern where TMF documents are filed late in a study, with significant upticks only at the end or during inspections, instead of a linear, contemporaneous filing. * **TMF QC Pass/Fail Calculation:** An example of how to create and calculate a quality score for each study by tracking passes and fails, allowing for ongoing assessment of TMF health and identification of areas for improvement. * **Subject Questionnaire QC Process:** A detailed walkthrough of how to perform a true review for a specific document type (subject questionnaires), considering protocol information, required iterations, translations, and IRB approvals.

Life at Veeva: Toronto
Veeva Systems Inc
/@VeevaSystems
Sep 27, 2018
This video serves as an internal recruitment and branding piece highlighting the corporate culture and strategic importance of Veeva Systems’ Toronto product hub. The primary goal is to showcase the employee experience, emphasizing the collaborative environment, the high caliber of colleagues, and the direct impact of their work on the life sciences industry. The Toronto office is positioned as Veeva’s fastest-growing product center, driving innovation by building end-to-end applications and leading the company's expansion into new, non-pharmaceutical sectors. The core themes articulated by the employees revolve around professional autonomy, speed, and purpose. Employees value the control they have over their work and the fast pace ("the speed that Veeva gives its employees... it keeps you on your toes"). A strong emphasis is placed on collaboration, defining the working environment as one where individuals function as partners and a unified team to find the best solutions for customers and product development. This collaborative, high-velocity culture is presented as essential for maintaining market leadership and driving continuous product evolution. Crucially for the life sciences sector, the video connects the technical work of software development directly to patient outcomes. Employees express motivation derived from "making a difference actually helping patients around the world." They explicitly state that Veeva’s software and services "speed up the process of getting the drugs to the market." This highlights Veeva's foundational mission—accelerating the delivery of life-saving treatments—which permeates the internal culture and serves as a powerful driver for talent acquisition and retention. The organizational structure is characterized by high-functioning, small teams operating within the framework of a large company. This structure allows for close collaboration and personal recognition while maintaining the resources and reach of a global enterprise. Furthermore, the company promotes continuous skill enhancement through specific initiatives, such as the "Two-Percent Development Program," which provides employees with opportunities to learn new skills and work on "new and upcoming" technologies. This commitment to professional growth ensures that the Toronto hub remains at the forefront of technological innovation, supporting both current product suites and the strategic expansion into new industries mentioned in the video's description. Key Takeaways: • **Strategic Importance of the Toronto Hub:** The Toronto office is explicitly identified as Veeva’s fastest-growing product hub, responsible for driving innovation in end-to-end applications and leading the company’s strategic expansion into new industries beyond its traditional life sciences focus. • **High-Velocity Development Environment:** Veeva maintains a culture defined by speed and pace, which keeps employees engaged and focused on rapid delivery. This pace is a critical factor for consulting partners like IntuitionLabs.ai to consider when planning integration and customization projects, as Veeva’s core platform updates and feature releases are likely frequent. • **Autonomy and Control in Product Development:** Employees emphasize the high degree of autonomy and control they possess over their work, suggesting a decentralized decision-making process that empowers developers to innovate quickly and respond directly to customer needs. • **Direct Link to Patient Impact:** The company culture strongly reinforces the connection between software development and real-world results, specifically "speed[ing] up the process of getting the drugs to the market" and "helping patients around the world." This mission alignment is a key driver for talent and reinforces Veeva’s value proposition to pharmaceutical clients. • **Collaborative Customer-Centric Approach:** The development environment is highly collaborative, with employees working as "partners" and a "team" to find the "best solution for our customers and our product," indicating a strong focus on user experience and practical utility in their software design. • **Small Team Structure for Agility:** Despite being a large company, Veeva operates using "really small teams," which fosters close working relationships, encourages individual contribution, and likely contributes to the high speed and agility of their product development cycles. • **Commitment to Continuous Skill Development:** The existence of the "Two-Percent Development Program" highlights Veeva’s investment in ensuring its technical staff are constantly acquiring new skills and working on emerging technologies, which is vital for maintaining the competitive edge of the Veeva platform ecosystem. • **Focus on Innovation and Upcoming Technologies:** The development program specifically focuses on working on things that are "new and upcoming," signaling that Veeva is actively investing in future-proofing its platform, potentially incorporating advanced technologies like AI/LLMs, which aligns directly with IntuitionLabs.ai’s core service offerings. Tools/Resources Mentioned: * **Veeva CRM:** Implied as a core product suite, given the company's focus on accelerating drug market access. * **Two-Percent Development Program:** An internal resource dedicated to employee skill development and exposure to new technologies. Key Concepts: * **End-to-End Applications:** Refers to software solutions that manage an entire process or workflow within the life sciences value chain, from clinical trials to commercial operations. * **Product Hub:** A strategic location dedicated to the research, design, and development of a company's software products, indicating a major investment in regional growth and talent acquisition. * **Accelerating Drug Time-to-Market:** The core business value proposition of Veeva, achieved by providing efficient, compliant, and integrated software solutions for pharmaceutical operations.

Meet Veeva Toronto: Software Engineer Edition
Veeva Systems Inc
/@VeevaSystems
Sep 27, 2018
The video offers a concise, internal perspective on the software development lifecycle and engineering culture within Veeva Systems, featuring a software engineer from the Toronto office. The primary purpose is to showcase the role of engineering in developing "next-generation technologies" specifically designed to transform the life sciences industry. This glimpse into Veeva’s operational methodology is crucial for partners like IntuitionLabs.ai, as it reveals the standards of quality, collaboration, and ownership expected within the core platform provider. The speaker, Suchintan Singh, emphasizes that the engineer’s role extends far beyond coding; it involves taking complete ownership of the product. This ownership encompasses the entire development pipeline, starting with deep engagement with Product Managers (PMs). The engineer is responsible for ensuring that the product requirements are "fully fleshed out," necessitating strong communication and a proactive approach to translating business needs into technical specifications. This initial phase is critical for preventing scope creep and ensuring the final product aligns perfectly with the needs of the highly regulated pharmaceutical and biotech sectors. Following implementation, the engineer’s ownership continues through rigorous collaboration with the Quality Assurance (QA) team. This partnership ensures that the implemented solution is robust, meets all defined requirements, and is ready for deployment. The central theme articulated is the pivotal role of seamless communication across all functions—PM, Engineering, and QA—as the foundational element for success. This integrated, quality-focused approach confirms Veeva’s commitment to delivering enterprise-grade software that can withstand the stringent regulatory demands of the life sciences industry, directly impacting how IntuitionLabs.ai must approach its own custom development and integration projects. Key Takeaways: * **Emphasis on Full Product Ownership:** Veeva’s engineering culture mandates that software engineers take complete, end-to-end ownership of their products, managing features from initial requirements gathering through implementation and final quality assurance. This model promotes accountability and deep domain understanding among technical staff. * **Critical Three-Way Collaboration:** The core development process relies on a tight feedback loop involving Product Managers (defining the 'what'), Software Engineers (building the 'how'), and Quality Assurance (validating the solution). IntuitionLabs.ai should adopt this structured, cross-functional approach in its own custom software development projects for life sciences clients. * **Communication as a Success Pivot:** The video explicitly states that good communication is "pivotal to our success," particularly in coordinating requirements with PMs and validating solutions with QA. This underscores the necessity for IntuitionLabs.ai to maintain exceptionally clear and documented communication when integrating with or customizing the Veeva platform. * **Focus on Next-Generation Technology:** Veeva engineers are actively developing advanced technologies aimed at changing the life sciences industry, confirming the platform’s continuous evolution and investment in areas relevant to IntuitionLabs.ai's focus on AI, LLMs, and intelligent automation. * **Rigorous Quality Assurance Standard:** The requirement for solutions to be "completely fully fleshed out" through collaboration with QA highlights Veeva's high standards for quality and stability, which is essential for maintaining GxP and 21 CFR Part 11 compliance in pharmaceutical operations. * **Proactive Requirements Refinement:** Engineers are expected to engage with PMs to ensure requirements are fully detailed, suggesting an iterative and consultative approach to specification development rather than passive execution. IntuitionLabs.ai consultants should emulate this proactive stance when scoping client projects. * **Strategic Investment in Engineering Hubs:** The video highlights the Toronto office as a key engineering location, providing insight into Veeva’s strategic geographic investments and talent acquisition focus for future platform development. * **Implication for AI/LLM Integration:** As Veeva continuously innovates its core platform, IntuitionLabs.ai must stay current with these "next-generation technologies" to ensure its custom AI and LLM solutions are built on stable, forward-compatible Veeva architecture. Tools/Resources Mentioned: * Veeva Platform (Implicit) Key Concepts: * **Product Manager (PM):** The role responsible for defining the product requirements, business goals, and overall strategy for a feature or product line. * **Quality Assurance (QA):** The systematic process of checking whether a product or service being developed is meeting specified requirements, particularly crucial in regulated environments like life sciences to ensure compliance and reliability. * **Product Ownership:** A development methodology where an individual or team is fully accountable for the success, quality, and maintenance of a specific product or feature throughout its entire lifecycle.