Videos
Browse videos by topic
All Videos
Showing 817-840 of 1435 videos

Webinar Core eTMF Features and Practices
Cloudbyz
/@cloudbyz
Apr 18, 2022
This webinar provides an in-depth exploration of Electronic Trial Master File (eTMF) features and best practices, crucial for anyone involved in clinical trials. Presented by subject matter experts from Cloudbyz, the session begins by defining what a Trial Master File (TMF) is, emphasizing its role as a repository for essential documents in a clinical research study, vital for efficient trial management and reconstruction during audits or inspections. The discussion then transitions to the significant advantages of an eTMF over traditional paper-based systems, highlighting how digital solutions address security, accessibility, and efficiency challenges inherent in managing vast amounts of critical trial documentation. The core of the presentation delves into the essential features required in an effective eTMF system. Speakers meticulously outline functionalities such as automated and configurable metadata capture, adherence to TMF reference models like the DIA standard, robust access permissions based on user roles, advanced search capabilities, and streamlined approval workflows. Furthermore, the webinar stresses the importance of efficient document upload mechanisms, comprehensive audit trails, timely notifications, and actionable reports and dashboards for assessing the health and completeness of the eTMF. A significant portion is dedicated to the critical aspect of regulatory compliance, detailing requirements from ICH GCP, European Union directives (including the 25-year archiving rule), US FDA regulations (21 CFR Part 11, Part 312, Part 812), and HIPAA. The experts also provide practical guidance on selecting the right eTMF vendor, advising attendees to consider factors beyond just cost, such as ease of use, training support, the completeness of core features, compliance adherence, scalability for future needs, and the vendor's quality as a true partner. The Q&A segment further enriches the discussion, addressing common concerns like custom eTMF structures, quality review items for study binders, the distinction and integration possibilities between eTMF and Electronic Investigator Site Files (eISF), and best practices for data privacy and security within eTMF systems. The overall perspective is one of practical application, aiming to equip clinical trial professionals with the knowledge to optimize their document management processes, ensure compliance, and enhance operational efficiency through electronic solutions. Key Takeaways: * **Definition and Purpose of TMF/eTMF:** A Trial Master File (TMF) is a repository of essential documents for a clinical research study, crucial for efficient trial management and reconstruction during audits or inspections. An eTMF is the electronic version, maintaining the same requirements as paper but with enhanced capabilities. * **Benefits of eTMF over Paper TMF:** Electronic systems offer superior security (user accounts, secure passwords, protection against data loss/alteration), remote access from anywhere, restricted role-based access with audit trails (critical for 21 CFR Part 11 compliance), efficient document search, and simplified archiving/retention without physical space constraints. * **Core eTMF Features for Efficiency:** Essential features include automated and configurable metadata for accurate data entry and reporting, support for TMF reference models (e.g., DIA) for standardized folder structures, granular access permissions, robust search functionalities, customizable approval workflows, and drag-and-drop document upload with holding areas for QC. * **Regulatory Compliance is Paramount:** eTMFs must comply with universally accepted guidelines like ICH GCP E6(R2) Chapter 8, EU directives (including the mandatory 25-year archiving post-trial), US FDA regulations (21 CFR Part 11 for electronic records/signatures, Part 312/812 for drug/device studies), and HIPAA for patient privacy. * **Importance of TMF Reference Models:** The DIA Reference Model is widely used for standardizing TMF content, providing a unified interpretation of regulations, defining required metadata, listing core and recommended documents, and addressing variability across different study types (e.g., drug vs. device). * **Strategic eTMF Vendor Selection:** Beyond cost, evaluate vendors based on ease of use, comprehensive training, the presence of critical core features, compliance capabilities, ease of collaboration with sites (e.g., eISF integration), implementation time, scalability for future growth, and the vendor's quality as a long-term partner. * **eTMF vs. eISF and Integration:** An eTMF manages trial-level documents, while an eISF (Electronic Investigator Site File) replaces physical site binders. They can be separate or combined; sponsors can provide sites access to specific eTMF folders, but sponsors typically do not have direct access to site-specific documents only accessible to CRAs. * **Achieving "Inspection Readiness":** An eTMF should always be inspection-ready, meaning all essential documents up to a given milestone (as defined by TMF plans or DIA model) are present, reviewed, correct, and have complete metadata. Automation in eTMFs can generate reports on missing documents, significantly simplifying this process compared to paper TMFs. * **Privacy and Security Best Practices:** Implement role-based access controls (especially for blinded/unblinded roles), ensure individual login credentials (no sharing), track user access and time, maintain comprehensive audit trails, and ensure regular backups of essential documents, ideally with secure data centers. * **Migration and Integration of eTMFs:** Migrating eTMFs between vendors or from electronic drives to dedicated systems is possible. Key quality checks include matching TMF indices, performing a complete audit for missing documents, and ensuring metadata is accurately captured and transferred to maintain document attributes and reporting capabilities. * **Significant Time and Cost Savings:** Digital eTMF and eISF systems can save up to 50% or more in time and effort, especially by enabling remote access for sites and monitors, reducing physical shipping costs, and streamlining document review processes. This also significantly reduces monitoring costs, which are a major expense in clinical trials. * **Customization of eTMF Structures:** While reference models like DIA provide a strong base, eTMF structures can be customized to fit specific study types (e.g., omitting bioanalytical report folders if not applicable). The key is to ensure the customized structure still contains all essential documents required by ICH GCP and other relevant regulations. **Tools/Resources Mentioned:** * Cloudbyz (eTMF platform) * ICH GCP E6(R2) (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Good Clinical Practice guidelines) * DIA Reference Model (Drug Information Association TMF Reference Model) * OS's reference model (another TMF reference model) * FDA 1572 (Form 1572, Statement of Investigator for drug studies) * 21 CFR Part 11 (Code of Federal Regulations, Title 21, Part 11 - Electronic Records; Electronic Signatures) * 21 CFR Part 312 (Investigational New Drug Application) * 21 CFR Part 812 (Investigational Device Exemptions) * HIPAA (Health Insurance Portability and Accountability Act) **Key Concepts:** * **TMF (Trial Master File):** A collection of essential documents that individually and collectively permit the evaluation of the conduct of a clinical trial and the quality of the data produced. * **eTMF (Electronic Trial Master File):** A TMF maintained electronically, offering enhanced features like security, remote access, and automation. * **eISF (Electronic Investigator Site File):** The electronic equivalent of the Investigator Site File, containing site-specific essential documents. * **ICH GCP:** International ethical and scientific quality standard for designing, conducting, recording, and reporting trials that involve the participation of human subjects. * **DIA Reference Model:** A standardized, industry-accepted content list and organizational structure for the TMF, facilitating consistency and compliance. * **Metadata:** Data that describes other data; in eTMF, it includes attributes like document type, version, date, and author, crucial for search and reporting. * **Audit Trail:** A chronological record of system activities, showing who did what, when, and where, essential for regulatory compliance (e.g., 21 CFR Part 11). * **21 CFR Part 11:** FDA regulation governing electronic records and electronic signatures, ensuring their trustworthiness, reliability, and equivalence to paper records. * **GxP:** A general term for "good practice" quality guidelines and regulations in various regulated industries, including Good Clinical Practice (GCP).

Patient Must Come First in Value Based Care
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Apr 16, 2022
This video provides an in-depth exploration of Value-Based Care (VBC) and the critical, often overlooked, imperative to prioritize the patient within this evolving healthcare model. Dr. Eric Bricker of AHealthcareZ discusses an article by Dr. Sachin Jain, CEO of SCAN Health, a non-profit Medicare Advantage plan, who brings extensive experience from government, major carriers, and direct patient care. The video establishes that while VBC is frequently praised in theory, its practical implementation often reverts to cost-containment strategies reminiscent of past managed care models, potentially sidelining patient well-being. The core of the discussion revolves around Michael Porter's value equation—quality divided by cost—and how VBC aims to increase quality while decreasing cost. However, Dr. Jain's enumerated seven tenets of VBC reveal a more complex reality. These tenets include stringent management of inpatient bed-days, mandatory specialist referrals requiring prior authorization, the use of narrow networks that may prioritize cost over specialist quality, and team-based care that can introduce additional gatekeepers before a patient sees a physician. Furthermore, VBC often involves low-cost formularies that limit access to newer, potentially expensive medications, an emphasis on addressing social determinants of health (though without robust population-level evidence of effectiveness), and ultimately, a focus on the VBC plan's own revenue and bottom line. Dr. Bricker highlights the striking similarities between these VBC tenets and the much-criticized HMO models of the 1990s, which faced significant backlash for failing to put patients first. To counteract this tendency and genuinely embed patient-centricity into VBC, Dr. Bricker proposes three structural and incentive-based solutions. These include placing physicians on salary with transparent variable compensation to remove financial incentives for delivering more or less care, establishing a third-party ombudsman or rapid response team (RRT) hotline for patients and healthcare staff to report issues of denied care or bureaucratic hurdles, and mandating that all professionals involved in designing or administering VBC plans (from doctors to politicians) must themselves be enrolled in a VBC insurance plan. These suggestions emphasize transparency and accountability, drawing parallels to the "sunlight is the best disinfectant" principle and Toyota's quality improvement process where any assembly line worker can halt production to address a problem. Key Takeaways: * **Value-Based Care (VBC) Definition:** VBC is theoretically defined by the value equation: Quality divided by Cost. The goal is to improve patient outcomes (quality) while reducing healthcare expenditures (cost). * **Practical Tenets of VBC:** Dr. Sachin Jain outlines seven practical tenets of VBC, which often prioritize cost control: managing inpatient bed-days, requiring specialist referrals, utilizing narrow networks, implementing team-based care, employing low-cost formularies, addressing social determinants of health, and focusing on the VBC plan's revenue. * **Cost Containment Strategies:** VBC models often involve aggressive strategies to reduce costs, such as minimizing hospital stays, requiring prior authorizations for specialist visits, and restricting choices of providers through narrow networks, which can sometimes override quality considerations. * **Gatekeeping in Care Delivery:** Team-based care, while beneficial in some aspects, can also create additional layers of gatekeepers (e.g., nurse practitioners before PCPs, PCPs before specialists), potentially delaying or complicating patient access to specialized care. * **Impact on Pharmaceutical Access:** Low-cost formularies are a critical component of VBC, limiting patient access to newer, often more expensive, medications. This directly impacts pharmaceutical companies' market access strategies and patient treatment options. * **Social Determinants of Health (SDOH):** While addressing SDOH is a VBC tenet, the video notes a lack of population-level studies proving its effectiveness, though individual cases (like providing a refrigerator for insulin storage) demonstrate its potential impact. * **Historical Parallels to HMOs:** The speaker draws a strong comparison between the current practical implementation of VBC and the much-criticized Health Maintenance Organizations (HMOs) of the 1990s, which faced backlash for not prioritizing patients. * **Ethical Imperative of Patient-First:** The fundamental ethical principle of "the patient comes first," ingrained in medical education, must serve as the guiding framework for all VBC strategies to prevent a repeat of past managed care failures. * **Physician Compensation Reform:** A proposed solution involves putting physicians on salary with transparent variable compensation. This aims to remove financial incentives that might lead doctors to provide either too much or too little care, allowing clinical judgment to be paramount. * **Third-Party Ombudsman for Accountability:** Implementing a third-party ombudsman or a "rapid response team" (RRT) hotline allows patients, doctors, and other healthcare team members to report issues like denied care, bureaucratic hurdles, or lack of access, fostering transparency and external scrutiny. * **"Eat Your Own Cooking" Principle:** A key recommendation is that all individuals involved in designing, administering, or regulating VBC (including doctors, administrators, insurance employees, and government officials) should be enrolled in VBC insurance plans themselves. This ensures they experience the system firsthand and are incentivized to create a patient-centric model. * **Transparency as a Disinfectant:** The overarching theme for proposed solutions is transparency, echoing Louis Brandeis's sentiment that "sunlight is the best disinfectant." Openness about compensation structures and avenues for reporting issues can drive accountability and patient-centricity. Key Concepts: * **Value-Based Care (VBC):** A healthcare delivery model where providers are paid based on patient health outcomes, rather than the volume of services provided. * **Value Equation (Quality / Cost):** A framework, popularized by Michael Porter, for defining value in healthcare as the health outcomes achieved per dollar spent. * **Medicare Advantage Plan:** A type of Medicare health plan offered by a private company that contracts with Medicare to provide all your Part A and Part B benefits. * **Formulary:** A list of prescription drugs covered by a health insurance plan. "Low-cost formularies" typically prioritize generic or less expensive brand-name drugs. * **Narrow Network:** A health insurance plan that restricts patients to a limited number of doctors and hospitals, often to control costs. * **Social Determinants of Health (SDOH):** Non-medical factors that influence health outcomes, such as socioeconomic status, education, neighborhood and physical environment, employment, and social support networks. * **Rapid Response Team (RRT):** A team of healthcare professionals in hospitals who respond to patients whose condition is deteriorating, before a full cardiac arrest or respiratory failure occurs. The video suggests an outpatient version for VBC issues. * **Ombudsman:** An official appointed to investigate individuals' complaints against maladministration, especially that of public authorities. * **Fee-for-service:** A payment model where providers are paid for each service they perform (e.g., office visit, test, procedure). Examples/Case Studies: * **SCAN Health:** Dr. Sachin Jain's non-profit Medicare Advantage plan in Southern California, serving as a real-world example of a VBC organization. * **Diabetic Patient and Refrigerator:** An example of addressing a social determinant of health where Dr. Jain's health plan purchased a refrigerator for a diabetic patient to properly store insulin. * **1990s HMOs:** The video frequently references the historical backlash against HMOs in the 1990s due to their cost-cutting measures leading to patient dissatisfaction, drawing a parallel to current VBC practices. * **Toyota Quality Improvement Process:** The analogy of Toyota's "Andon cord" system, where any worker can stop the assembly line to address a quality issue, is used to illustrate the concept of a rapid response team or ombudsman in VBC.

eTMF System Specific Training (Veeva Vault)
Power of Work
/@powerofwork6914
Apr 13, 2022
This video provides specific training on the Veeva Vault eTMF (electronic Trial Master File) system, focusing on its features and functionalities for managing clinical trial documentation within the life sciences industry. The speaker, from Power of Work, guides viewers through navigating the system, applying filters, viewing documents, and critically, generating reports. The discussion highlights the transition from paper-based TMFs to eTMFs, emphasizing the significant advantages Veeva Vault offers in terms of regulatory compliance, data visibility, collaboration, and security. It also touches upon the system's role in supporting TMF specialists and its broader integration within the Veeva ecosystem. Key Takeaways: * **Veeva Vault as a Prevalent eTMF Solution:** Veeva Vault is presented as a widely adopted, comprehensive, cloud-based eTMF platform, particularly suited for large pharmaceutical companies due to its extensive features and the robust IT support required for its maintenance and updates. * **Ensuring Regulatory Compliance:** The video underscores the critical role of eTMF systems like Veeva Vault in maintaining regulatory compliance, specifically mentioning 21 CFR Part 11, electronic signatures, and audit trails. It emphasizes that a complete and accurate TMF is essential for successful FDA and other regulatory agency inspections. * **Enhanced Data Visibility and Reporting Capabilities:** Veeva Vault significantly improves operational efficiency by offering advanced filtering and reporting functions. These allow users to gain real-time insights into TMF completeness, track document processing, and monitor team performance, which is crucial for proactive management and audit readiness. * **Secure Collaboration and Audit Trails:** The system facilitates secure external collaboration, enabling multiple stakeholders to access and contribute to the TMF with defined roles and permissions. Its robust audit trail functionality ensures accountability and traceability for all document interactions, enhancing data integrity. * **Strategic Importance of Metadata Management:** The training highlights that a key aspect of TMF quality control involves cross-referencing document content with its associated metadata within the system, stressing the necessity for accurate data entry and consistency for effective document retrieval and reporting. * **Integration within the Life Sciences Technology Landscape:** The video notes Veeva Vault's evolving integration capabilities, including its use as a Clinical Trial Management System (CTMS), indicating its broader role and interconnectedness within the suite of technology solutions utilized in clinical research.

Planisware Academy Teaser
Planisware
/@Planisware
Apr 13, 2022
This video serves as a promotional teaser for the Planisware Academy, an official e-learning platform designed to educate Planisware customers and partners on the capabilities and usage of Planisware Products, specifically Planisware Enterprise. The core purpose of the Academy is to provide structured, comprehensive training aimed at achieving high user proficiency and formal certification across various skill levels. This initiative signals a strong institutional commitment by Planisware to ensuring their enterprise software is utilized effectively by their complex client base, which heavily includes pharmaceutical and life sciences organizations managing large R&D and clinical portfolios. The course structure is built around an interactive map that guides users through different learning paths, centered on practical application via "user stories." These scenarios follow an imaginary "Acme client" as they navigate typical operational challenges within the Planisware environment. In each scenario, the client expresses a specific need related to a topic (e.g., resource allocation, project tracking, budget management). Planisware experts then offer a detailed solution and explanation of the underlying process. This methodology ensures that learning is grounded in real-world operational context, allowing users to immediately connect theoretical knowledge with practical enterprise workflows. To enhance the learning experience, most courses incorporate step-by-step video tutorials, catering to visual learners and providing detailed guidance necessary to master complex subjects within the platform. Knowledge retention is reinforced through quizzes administered at the conclusion of each course module. The entire curriculum is organized into four distinct levels of difficulty: Associate, Contributor, Specialist, and Expert. Progression through these levels culminates in a final examination, upon successful completion of which the user obtains a formal certification, establishing recognized expertise in the Planisware Enterprise platform. This structured certification path indicates the depth of knowledge required to effectively manage enterprise-level portfolio and resource management within the life sciences sector. Key Takeaways: • **Validation of Enterprise Focus:** The existence of a dedicated, highly structured Planisware Academy confirms the platform's critical role as an enterprise-level system within the pharmaceutical and life sciences sectors, particularly for R&D and Project Portfolio Management (PPM). • **Mapping AI Solutions to User Stories:** IntuitionLabs.ai can leverage the "user story" methodology described in the training to identify common pain points and operational needs that Planisware users face, allowing for precise targeting and integration of custom AI and LLM solutions (e.g., automating data entry, predictive resource modeling). • **Benchmarking Client Expertise:** The four-tiered certification structure (Associate, Contributor, Specialist, Expert) provides a clear benchmark for assessing the internal capabilities of client teams. This is crucial for IntuitionLabs when scoping consulting projects, data engineering initiatives, or system integration efforts, ensuring solutions are tailored to the organization's existing expertise level. • **Opportunity for Integration Consulting:** The need for extensive, structured training, including step-by-step video tutorials, suggests that Planisware Enterprise is a complex system. This complexity often creates demand for specialized consulting services focused on advanced customization, system integration with other platforms (like Veeva CRM or clinical data systems), and building robust data pipelines—all core services offered by IntuitionLabs. • **Data Pipeline Relevance:** Planisware, as a PPM tool, holds vast amounts of critical data regarding project timelines, resource allocation, and budget expenditure. IntuitionLabs' data engineering services are essential for extracting, cleaning, and integrating this data with other enterprise systems to create comprehensive business intelligence dashboards and feed AI models. • **Focus on Practical Application:** The course design, which moves from client need to expert solution and detailed process explanation, emphasizes practical application over theoretical knowledge. IntuitionLabs should ensure its own AI solutions are marketed and implemented with a similar focus on solving immediate, practical operational challenges identified within the Planisware ecosystem. • **Partnership Potential:** As the Academy is open to partners, this suggests a formal channel for IntuitionLabs to engage with Planisware, potentially leading to joint solution development or co-marketing efforts focused on AI-powered enhancements for portfolio management within the life sciences industry. Tools/Resources Mentioned: * Planisware Enterprise * Planisware Academy (e-learning platform) Key Concepts: * **User Stories:** A core instructional methodology where learning is based on solving specific, real-world operational challenges experienced by a fictional client, ensuring practical relevance. * **Certification Levels:** A structured system for validating user proficiency, categorized into four tiers: Associate (basic knowledge), Contributor, Specialist, and Expert (deep platform mastery). * **Interactive Map:** The navigational structure of the e-learning platform, designed to guide users through sequential learning paths based on specific topics and user roles.

Dependent Audit - Are the People on your Health Plan Actually Eligible?
Self-Funded
@SelfFunded
Apr 11, 2022
This video provides an in-depth exploration of dependent eligibility audits, a critical cost containment and fiduciary responsibility mechanism for self-funded employers. The discussion, featuring Keith Bird of AmWINS Group Benefits, defines the audit as a process to verify that dependents enrolled on an employer-sponsored health plan are genuinely eligible according to the Summary Plan Description (SPD). The central motivation for conducting these audits is financial, driven by the realization that an estimated 3% to 6% of dependents on average are ineligible, often due to misunderstandings, status changes (like divorce), or intentional fraud. This leakage represents a significant and unnecessary drain on the employer’s healthcare budget. The conversation heavily emphasizes the financial return on investment (ROI) and the critical risk mitigation aspects of the audit. Statistically, the average savings from removing a single ineligible dependent is estimated conservatively at $4,500, leading to an overall ROI of 8 to 10 times the cost of the audit. Beyond direct cost savings, the most severe risk addressed is the potential voidance of stop-loss insurance coverage. If an ineligible dependent incurs a multi-million dollar claim, the stop-loss carrier will check eligibility, and if the dependent is found to be non-compliant with the SPD, the employer may be liable for the entire claim, underscoring the fiduciary duty under ERISA to maintain plan integrity. Technologically, the process has evolved significantly from cumbersome paper-based mailings to streamlined digital platforms. Modern audits leverage websites and mobile applications, allowing employees to answer relationship questions and upload necessary documentation (like marriage certificates or tax forms) via their smartphones. This technological shift has dramatically reduced the friction and pushback associated with the audit process, which the speaker notes is surprisingly low (less than 1% of employees typically protest). Furthermore, the audit process serves as a crucial catalyst for improving the plan’s foundational document. Approximately 80% of plan sponsors make adjustments to their SPD language after the audit team reviews the existing rules, tightening up ambiguous language regarding relationships such as common law marriage, domestic partnerships, or foster children, ensuring clarity and defensibility. Key Takeaways: • **Significant Cost Leakage:** Employers should anticipate that 3% to 6% of enrolled dependents are ineligible, representing a substantial, unnecessary cost burden on self-funded health plans. • **High Financial ROI:** The average savings from removing one ineligible dependent is conservatively estimated at $4,500, yielding an expected return on investment (ROI) of 8 to 10 times the audit cost. • **Critical Stop-Loss Risk Mitigation:** Failure to police dependent eligibility exposes the employer to catastrophic financial risk, as stop-loss carriers will check eligibility and may refuse to reimburse multi-million dollar claims incurred by ineligible individuals. • **ERISA Fiduciary Responsibility:** Maintaining plan integrity and ensuring only eligible participants receive benefits is a core fiduciary duty under ERISA, making dependent audits a necessary compliance measure, not just a cost-saving exercise. • **SPD Language Clarification is Essential:** The audit process typically reveals poorly written or ambiguous Summary Plan Description (SPD) language. Approximately 80% of clients modify their SPD after the audit review to clearly define inclusive and exclusive dependent relationships (e.g., foster children, common law spouses). • **Technology Drives Adoption:** Modern audits utilize technology (websites, mobile apps, photo uploads) to simplify the document submission process for employees, significantly reducing administrative burden and minimizing employee pushback, which is typically less than 1%. • **Ongoing Monitoring is Recommended:** While a one-time audit provides immediate savings, large organizations with high attrition or frequent status changes benefit from continuous monitoring of new hires, open enrollment changes, and spousal surcharges to maintain plan integrity. • **Spousal Carve-Outs and Surcharges:** Audits can effectively police spousal carve-out rules (requiring spouses with coverage elsewhere to take it) and surcharge requirements, generating significant revenue or savings (e.g., one client saved $45 million by enforcing a carve-out). • **Avoid Internal Audits:** Attempting a comprehensive dependent audit internally is highly discouraged, as companies lack the specialized technology and objective third-party distance required, often resulting in a "train wreck" due to complexity and internal friction. • **Impact of Healthcare Costs on Behavior:** The high cost of healthcare motivates some individuals to intentionally skirt the rules; one example cited was an employee who quickly married their partner after being notified they were ineligible to keep their benefits. Key Concepts: * **Dependent Eligibility Audit:** A formal, third-party review process conducted by self-funded employers to verify that all enrolled dependents (spouses, children, etc.) meet the eligibility criteria defined in the Summary Plan Description (SPD). * **Summary Plan Description (SPD):** The legal document that serves as the "bible" for a self-funded plan, detailing all rules, including who is eligible for coverage. Clarity in this document is paramount for audit success and legal defense. * **Stop-Loss Insurance:** Coverage purchased by self-funded employers to protect against catastrophic claims. If a claim exceeds the stop-loss deductible, the carrier will verify dependent eligibility before reimbursement, making the audit crucial for risk management. Examples/Case Studies: * **30-Year Ineligible Dependent:** The first dependent removed in a pilot audit had been on the plan for 30 years under a common law marriage status not covered by the plan, resulting in $256,000 in claims paid that should have been avoided. * **HR Manager Fraud:** An HR manager who knew the rules was caught covering her ex-spouse for three years and subsequently lost her job, illustrating the consequences of intentional abuse. * **Shotgun Weddings:** Several instances were noted where employees, upon learning their spouse was ineligible and would be removed, quickly submitted marriage certificates dated just days prior to the deadline to retain coverage. * **Hospital Custody Rule Change:** A large hospital system had a rule covering children only during years when the employee had custody. After the audit generated a thousand phone calls from affected employees, the rule was immediately changed mid-cycle via a summary modification to prevent mass removals.

Veeva Black Community | Meet Brandon
Veeva Systems Inc
/@VeevaSystems
Apr 11, 2022
This video provides a brief, focused perspective from a Veeva professional regarding the critical role of organizational support and continuous professional development within the demanding tech landscape of the life sciences industry. The speaker, Brandon, a consultant specializing in Commercial Strategy at Veeva, emphasizes that feeling empowered and capable of taking on significant responsibility is inextricably linked to the availability of resources and a robust support ecosystem. This is particularly true in the technology sector, where proficiency requires constant re-evaluation, re-education, and the ability to pivot rapidly based on emerging trends. The core theme revolves around the necessity of professional alignment and continuous growth. Brandon articulates his personal commitment to staying educated and advancing professionally, noting that Veeva provides the necessary backing to meet these high standards. This internal support system allows him to challenge himself and pursue personal development goals, which directly translates into higher quality consulting work for the pharmaceutical and life sciences clients Veeva serves. This perspective highlights that successful technology implementation in regulated industries relies not just on technical skills, but on the sustained confidence and strategic insight of the consulting workforce. In his role as a Consultant for Commercial Strategy, Brandon stresses that he is empowered to "push the envelope" and establish a "credible stake on the industry." This suggests that Veeva consultants are viewed as strategic partners who actively shape the commercial operations landscape for pharmaceutical companies, rather than merely implementing software. The confidence instilled by the supportive ecosystem enables consultants to take ownership of complex, high-stakes projects, ensuring that commercial teams leverage the full potential of the Veeva platform—a crucial consideration for firms like IntuitionLabs that specialize in maximizing Veeva CRM investment through advanced AI integration and customization. The short testimonial underscores the high-pressure, high-value environment of pharmaceutical technology consulting. The constant need to re-educate and pivot on trends validates the business model of specialized consulting firms that must remain agile and integrate cutting-edge solutions, such as LLMs and advanced data engineering, into established platforms like Veeva CRM. The focus on Commercial Strategy confirms that this area remains central to the industry's digital transformation efforts, requiring consultants who are not only technically proficient but also strategically aligned with the client's commercial goals. ### Key Takeaways: * **Mandate for Continuous Professional Development:** The pharmaceutical technology sector demands constant re-evaluation, re-education, and the ability to pivot on trends. Consulting firms specializing in Veeva must prioritize continuous training in new features, regulatory changes, and emerging technologies (like Generative AI) to maintain relevance and expertise. * **Strategic Importance of Commercial Strategy Consulting:** The role of a Commercial Strategy consultant within the Veeva ecosystem is highly influential, enabling professionals to "own a credible stake on the industry." This confirms that Veeva implementations are viewed as strategic business drivers critical to market success in life sciences. * **Ecosystem Support Drives Confidence:** A strong organizational support system and access to resources are directly linked to a consultant's ability to feel empowered, take on greater responsibility, and instill confidence in their purpose. This is essential for managing complex, regulated projects where assurance and expertise are paramount. * **Innovation through Empowerment:** The desire to "push the envelope" indicates that successful consulting involves looking beyond standard configuration to find innovative solutions. This aligns with the value proposition of specialized firms offering custom software and AI solutions to optimize commercial processes within the Veeva framework. * **Resource Mapping is Critical:** The success of professional growth is linked to "available resources mapped to an ecosystem's support." For consulting partners, this means ensuring deep integration with Veeva's partner programs and having internal resources dedicated to data engineering and regulatory compliance. * **Alignment with Commercial Operations:** The specific focus on Commercial Strategy reinforces that this department is a primary target for high-value consulting services, including CRM optimization, sales force effectiveness, and data-driven business intelligence. * **High Demand for Agility:** The necessity to "re-educate and pivot on trends" highlights the volatile nature of the tech landscape, requiring consulting firms to build flexible service offerings that can quickly incorporate new AI models or regulatory requirements (e.g., automating compliance tracking). * **Value of Deep Industry Knowledge:** While technical proficiency is assumed, the ability to gain a "credible stake" suggests that deep industry knowledge—understanding GxP, 21 CFR Part 11, and FDA/EMA requirements—is essential for consultants to be seen as strategic advisors rather than just technical implementers. ### Key Concepts: * **Commercial Strategy Consulting:** Refers to the specialized consulting practice focused on optimizing the sales, marketing, and medical liaison activities within pharmaceutical and life sciences organizations, often leveraging platforms like Veeva CRM to drive efficiency and compliance. * **Support Ecosystem:** The network of resources, training, mentorship, and organizational backing provided by a platform vendor (Veeva) or an employer, which is necessary for professionals to maintain high performance and adapt to industry changes. * **Push the Envelope:** A term indicating a commitment to innovation, challenging existing processes, and implementing advanced solutions (such as AI agents or LLMs) to achieve superior operational outcomes.

High Cost Healthcare Claimants
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Apr 10, 2022
This video provides an in-depth exploration of the primary driver of high healthcare costs in employer-sponsored health plans: high-cost claimants. Dr. Eric Bricker, the speaker, begins by establishing the context that anyone involved in managing a health plan budget must constantly focus on these individuals. He highlights the well-known 80/20 rule, where 80% of healthcare costs are driven by just 20% of claimants (typically those exceeding $25,000 annually), and further emphasizes the 5/50 rule, where 50% of costs come from the top 5% of claimants (those over $100,000 per year). The core message is that addressing overall healthcare costs is impossible without directly confronting the issue of high-cost claimants. The presentation then delves into categorizing high-cost claimants, first by their primary diagnostic areas and then by their claims patterns. Dr. Bricker identifies three major diagnostic categories: musculoskeletal (MSK), cardiovascular, and cancer. He provides important nuances, such as including "injury and poisoning" within MSK for a more accurate spend analysis, and expanding "cardiovascular" to encompass diabetes and renal failure, as these often stem from the same underlying vascular pathologies. He stresses that daily attention to these three categories is crucial for effective cost management. Following the diagnostic breakdown, the video introduces a critical framework for understanding the heterogeneity of high-cost claimants, noting that two-thirds of them are new each year. Dr. Bricker outlines three distinct claims patterns: "The Explosion," representing about 50% of new high-cost claimants, characterized by sudden, catastrophic events with little to no prior claims (e.g., heart attack, stroke, new cancer diagnosis); "The Ramp-Up," accounting for approximately 17% of high-cost claimants, showing a gradual increase in costs over time (e.g., progression to dialysis for end-stage renal disease); and "The Continuation," making up about 33% of high-cost claimants, who were high-cost in the previous year and remain so (e.g., cancer recurrence, congestive heart failure, complex surgical complications). He concludes by discussing the three ways claimants exit the high-cost category: leaving the plan, expiring, or resolving their condition. Key Takeaways: • **High-Cost Claimants Drive the Majority of Healthcare Costs:** A disproportionate amount of healthcare spend is concentrated in a small percentage of claimants. Specifically, 80% of costs are driven by 20% of claimants (typically >$25K/year), and 50% by just 5% of claimants (typically >$100K/year). This concentration necessitates a focused strategy. • **Primary Diagnostic Categories:** The vast majority of high-cost claims fall into three main diagnostic areas: Musculoskeletal (MSK), Cardiovascular, and Cancer. Employers and benefits managers must prioritize understanding and addressing these specific health challenges. • **MSK Spend Includes Injury:** When analyzing musculoskeletal costs, it's crucial to include "injury and poisoning" diagnostic codes, as many long-term musculoskeletal issues are categorized under injury, which can be overlooked if only "MSK" codes are considered. • **Cardiovascular Spend Encompasses Diabetes and Renal Failure:** Cardiovascular costs extend beyond direct heart and stroke events to include diabetes and kidney failure. These conditions share common vascular damage pathologies, making their management integral to a comprehensive cardiovascular health strategy. • **Three Distinct Claims Patterns:** High-cost claimants are not a homogeneous group but follow three patterns: "The Explosion" (sudden, catastrophic events with no prior claims, ~50% of new high-cost claimants), "The Ramp-Up" (gradual increase in costs over time, ~17%), and "The Continuation" (ongoing high costs from previous years, ~33%). • **Challenges in Predicting "The Explosion":** Claims analytics are ineffective for "Explosion" type claimants because they have no prior claims data to analyze. This highlights a significant limitation of retrospective data analysis for a large segment of high-cost events. • **Primary Care as a Solution for "The Explosion":** For "Explosion" claimants, the most effective intervention is robust primary care. This can be delivered through various models (onsite, nearsite, direct primary care, virtual) and is essential for early detection and prevention before catastrophic events occur. • **Proactive Intervention for "The Ramp-Up":** Claims analytics are highly effective for identifying "Ramp-Up" claimants, such as those progressing towards renal failure due to diabetes or hypertension. Employers should aim for 100% identification of these individuals to enable timely and impactful interventions. • **Ongoing Management for "The Continuation":** Claimants in "The Continuation" category require sustained management and support due to chronic or recurring conditions like cancer recurrence, congestive heart failure, or complications from previous surgeries. • **Two-Thirds of High-Cost Claimants are New Annually:** The majority of high-cost claimants (two-thirds) are new each year, meaning strategies cannot solely focus on existing high-cost individuals but must also account for continuous new entries. • **Cut Through Distractions:** Benefits managers, HR, and CFOs must resist getting sidetracked by numerous health plan distractions (plan design, compliance, employee complaints, data issues) and maintain a daily focus on addressing high-cost claimants. • **Claimant Exit Strategies:** High-cost claimants exit the category through three main avenues: leaving the health plan (e.g., employee changes jobs), expiring, or resolving their medical condition (e.g., successful cancer treatment, kidney transplant). Key Concepts: * **High Cost Claimants:** Individuals whose healthcare claims exceed a specific annual financial threshold (e.g., $25,000 or $100,000), accounting for a disproportionately large share of total healthcare expenditures. * **80/20 Rule (Pareto Principle):** A general rule stating that roughly 80% of effects come from 20% of causes. In healthcare, this means 80% of costs are driven by 20% of claimants. * **5/50 Rule:** A more extreme variant of the Pareto Principle, indicating that 50% of healthcare costs are driven by just 5% of claimants. * **Diagnostic Categories:** Broad medical classifications used to group similar conditions, which in this context, highlight the primary areas of high healthcare spend (MSK, Cardiovascular, Cancer). * **Claims Patterns:** Distinct trajectories of healthcare utilization and cost accumulation over time, categorized as "The Explosion," "The Ramp-Up," and "The Continuation." * **Primary Care:** Comprehensive, first-contact healthcare that focuses on prevention, early detection, and management of common health problems, crucial for mitigating "Explosion" type high-cost events. Examples/Case Studies: * **"The Explosion" Examples:** Heart attack, stroke, or a new cancer diagnosis in an otherwise healthy individual (e.g., a 45-year-old with metastatic colon cancer appearing suddenly). * **"The Ramp-Up" Examples:** The progressive decline of kidney function leading to dialysis for End-Stage Renal Disease, often secondary to long-standing diabetes and hypertension. * **"The Continuation" Examples:** Recurrence or metastasis of cancer, ongoing management of congestive heart failure, complications from unsuccessful spine surgery, or the need for revision surgeries for joint replacements.

2022 Veeva R&D and Quality Summit
Veeva Systems Inc
/@VeevaSystems
Apr 8, 2022
The 2022 Veeva R&D and Quality Summit serves as a pivotal annual gathering for the life sciences industry, specifically targeting professionals involved in research, development, and regulatory compliance. The video captures the essential value proposition of the event, emphasizing its role as a critical platform for networking, knowledge exchange, and strategic alignment within the Veeva ecosystem. Attendees highlight that the primary benefit of the summit is the opportunity to connect with new customers and industry peers, fostering a collaborative environment where organizations can share their operational successes, challenges, and future initiatives. A core theme articulated by participants is the importance of learning from shared experiences and best practices. The summit functions as an incubator for innovation, where the discussion of current initiatives and successful deployments can "inspire us to do something new." This focus on practical, real-world application of technology and compliance strategies is essential for companies operating in the highly regulated pharmaceutical and biotech sectors. The event brings together diverse stakeholders, ensuring that the insights shared are relevant across the entire spectrum of the life sciences value chain, from early-stage research to quality management and regulatory submission. The composition of the audience underscores the summit's significance as a central industry meeting point. Attendees noted the presence of colleagues from "all walks of life," specifically mentioning representatives from large pharmaceutical companies ("big pharma"), medium-sized pharma organizations, and emerging "biological startups." Furthermore, the inclusion of organizations like Contract Research Organizations (CROs) ensures that the dialogue encompasses the entire outsourced R&D landscape. Strategically, the most valuable aspect highlighted is the opportunity for "very much close discussions directly with some of the product managers," a level of access that is typically unavailable through standard channels. This direct line of communication with Veeva product leadership is invaluable for consulting firms and end-users alike, allowing them to gain insight into future product roadmaps, provide feedback, and ensure their internal technology strategies remain aligned with Veeva’s platform evolution. Key Takeaways: * **Strategic Networking and Customer Acquisition:** The summit is identified as a prime venue for meeting potential new customers and strengthening relationships with existing clients within the pharmaceutical, biotech, and CRO sectors, validating its importance for business development and market penetration. * **Competitive Intelligence and Best Practice Sharing:** Attending organizations gain critical competitive intelligence by observing the initiatives and successful deployments shared by peers. This exchange of "best practices" helps firms benchmark their operational efficiency and identify areas for optimization, particularly concerning Veeva platform utilization. * **Inspiration for Innovation:** The exposure to diverse experiences and successful initiatives from various companies—ranging from startups to large enterprises—serves as a catalyst for internal innovation, prompting attendees to consider new approaches to R&D, Quality, and regulatory compliance. * **Comprehensive Industry Representation:** The event successfully aggregates the entire life sciences ecosystem, including big pharma, mid-sized companies, and biological startups. This broad representation ensures that the discussions and solutions presented are relevant across different scales and business models. * **CRO and Outsourcing Focus:** The presence of CROs (implied by the mention of industry partners) confirms the summit's relevance to the outsourced clinical and quality management space, highlighting the need for robust, compliant technology solutions across the partner network. * **Direct Access to Veeva Product Roadmap:** The ability to engage in "close discussions directly with some of the product managers" is a crucial strategic advantage. This access allows consulting firms to anticipate future platform changes, understand upcoming features, and tailor their service offerings (e.g., AI integration, data engineering) to align with Veeva's development trajectory. * **Validating Technology Investment:** For organizations specializing in Veeva consulting and AI solutions, the summit provides validation of the platform's continued importance in R&D and Quality, reinforcing the decision to invest heavily in specialized expertise for these regulated areas. * **Regulatory and Quality Focus:** Given the summit's R&D and Quality focus, the discussions inherently revolve around GxP compliance, data integrity, and 21 CFR Part 11 requirements, which are central to the operational and regulatory services offered to the target market. * **Understanding Customer Pain Points:** Direct interaction with end-users and product managers allows consultants to gain deeper insights into current customer pain points related to Veeva implementation, data migration, and system integration, enabling the development of more targeted and effective custom software and AI solutions. Tools/Resources Mentioned: * Veeva R&D and Quality Summit (2022) * Veeva Systems Inc. (Implied platform focus) Key Concepts: * **Best Practices Sharing:** The process of exchanging successful methods, strategies, and operational approaches among industry peers to improve collective performance and efficiency. * **Product Manager Access:** Direct, high-level engagement with the individuals responsible for defining the future features and direction of a software platform (Veeva), crucial for strategic alignment and competitive advantage. * **Industry Ecosystem:** The collective network of organizations—including pharmaceutical companies, biotech firms, CROs, and technology providers—that operate within the life sciences sector.

Define Regulatory Intelligence
Shurig Solutions Inc
/@shurigsolutionsinc6472
Apr 6, 2022
This video provides an in-depth exploration of Regulatory Intelligence, specifically differentiating between its compliance and policy aspects and emphasizing the critical importance of a proactive approach. The discussion begins by acknowledging that establishing a full-fledged Regulatory Intelligence department covering both compliance and policy might be resource-intensive. Therefore, it suggests that organizations, particularly those with limited bandwidth or financial resources, should prioritize focusing on the compliance component first. This foundational step is crucial for maintaining operational continuity, ensuring products remain on the market, and preventing costly emergencies. The core argument presented revolves around the stark contrast between proactive and reactive regulatory management. The speaker highlights that a reactive stance often leads to "oh crap moments," such as an auditor discovering a year-old regulatory change that the company failed to implement. Such oversights can result in severe consequences, including product recalls, mandatory additional testing, or even the inability to access certain markets. These reactive measures not only disrupt operations but also incur significant financial penalties, far exceeding the cost of proactive measures. The video stresses that companies will inevitably pay for regulatory adherence one way or another – either through strategic, upfront investment in prevention or through much larger, emergency-driven expenses on the back end. The discussion further illustrates the practical implications of proactive compliance, particularly in new product development. It underscores the necessity of integrating regulatory changes into the development process early on. Failing to do so could lead to a product being developed with incorrect testing protocols, such as an outdated IEC test, rendering it ineligible for specific markets a year down the line. The speaker's perspective is pragmatic, advocating for a focus on "keeping the doors open" and products viable through diligent, forward-looking regulatory intelligence. This approach is framed not just as a regulatory obligation but as a strategic imperative to prevent operational disruptions and safeguard financial health. Key Takeaways: * **Prioritize Compliance in Regulatory Intelligence:** When resources are limited, organizations should initially focus their Regulatory Intelligence efforts on the compliance aspect. This means concentrating on immediate operational needs like keeping products on the market and ensuring daily activities adhere to current regulations. * **Proactive vs. Reactive Regulatory Management:** A central theme is the necessity of shifting from a reactive to a proactive mode in response to regulatory changes. Waiting for an auditor to identify a non-compliance issue is a costly and detrimental approach. * **High Costs of Reactivity:** Being reactive to regulatory changes inevitably leads to "emergency" situations that incur significant financial and operational costs. These can include product recalls, mandatory re-testing, fines, and potential market access restrictions. * **Financial Investment in Proactivity:** Companies will pay for regulatory compliance regardless. Investing proactively in regulatory intelligence and prevention is presented as a more cost-effective strategy than paying for the consequences of non-compliance on the back end, which can lead to even greater financial losses. * **Operational Continuity:** Proactive compliance is essential for maintaining business operations, ensuring products remain available to consumers, and avoiding disruptions that can stem from regulatory breaches. * **Integrate Regulatory Changes Early in Development:** For new product development, it is crucial to incorporate regulatory changes into the system promptly. This ensures that the correct tests are performed at the right time, preventing future issues like products failing to meet market-specific standards (e.g., IEC tests). * **Continuous Monitoring of Standards and Regulations:** Effective regulatory intelligence involves constantly monitoring what standards and regulations are changing. Being aware of these shifts allows companies to adapt their processes and products before non-compliance becomes an issue. * **Preventing Emergencies:** The primary goal of proactive regulatory intelligence is to prevent emergencies rather than merely reacting to them. This involves anticipating potential issues and implementing preventative measures. * **Strategic Resource Allocation:** Organizations should consider where to allocate resources – either upfront for proactive intelligence and prevention, or later for damage control and remediation, with the former being the more strategic and financially prudent choice. Key Concepts: * **Regulatory Intelligence:** The process of gathering, analyzing, and disseminating information about regulatory changes, trends, and requirements to support strategic decision-making and ensure compliance. * **Compliance:** Adherence to established laws, regulations, guidelines, and specifications relevant to the industry and products. In the context of the video, it focuses on the immediate operational requirements to keep products on the market. * **Policy:** Refers to broader strategic regulatory considerations, which might involve influencing future regulations or long-term market access strategies, distinct from day-to-day compliance. Examples/Case Studies: * **Auditor Findings:** The scenario of an auditor discovering a year-old regulatory change that the company was unaware of, leading to immediate corrective actions. * **Product Recalls and Additional Testing:** Direct consequences of non-compliance, forcing companies to pull products from the market or conduct expensive, unplanned testing. * **New Product Development with Incorrect Testing:** A product developed without incorporating updated regulatory requirements (e.g., an IEC test) might be rendered ineligible for a target market a year after its development, highlighting the cost of oversight.

Surgeon Quality Scoring - Sanjay Prasad - Episode 40
Self-Funded
@SelfFunded
Apr 4, 2022
This video provides an in-depth exploration of surgeon quality scoring, patient empowerment in healthcare decisions, and the future role of technology, particularly AI, in optimizing surgical outcomes and costs. Dr. Sanjay Prasad, a neurotologist, head and neck cranial base surgeon, author of "Resetting Healthcare," and founder of SurgiQuality, discusses his mission to bring transparency to surgical care. He highlights the critical need for patients to make well-informed choices, challenging the current healthcare system where medical errors are the third leading cause of death and a significant percentage of surgeries are deemed unnecessary. Dr. Prasad traces his journey from a passionate microsurgeon to an innovator in healthcare transparency. He recounts his early experience opening an ambulatory surgery center in 2007 to save costs by bundling services (surgeon, facility, anesthesia fees). This initial focus on price evolved into SurgiQuality, founded in 2014, with the crucial addition of quality metrics to bundled rates. His methodology involves granular, procedure-specific quality measures, such as bile duct injury rates for gallbladder surgery or adenoma detection rates (ADR) for colonoscopies, which are validated using a proprietary AI-powered engine that analyzes electronic medical records. This system allows surgeons to market their high outcomes and patients to choose providers based on proven success rates and lower complication risks. The SurgiQuality platform targets self-insured employers, offering a concierge service that guides members through surgical decisions. When a member is told they need surgery, they sign a HIPAA release, and SurgiQuality's concierge collects medical records and imaging. This data is then sent to a network of surgeons who review the case, validate its necessity, and provide their past experience and outcome data. The AI engine scores these surgeons against peers and national norms, presenting the patient with a list of high-quality, cost-effective options within their network. This approach not only empowers patient choice but also aims to significantly reduce unnecessary surgeries—which Dr. Prasad estimates to be 15-30% across specialties, and as high as 80% for spine surgery—and mitigate stop-loss risk for employers by preventing catastrophic claims. He also envisions a future where digital technologies, wearables, and AI can predict health conditions and prevent major events like strokes or gallstones through early detection and lifestyle modifications. Key Takeaways: * **Prevalence of Medical Errors and Unnecessary Surgeries:** Medical errors are alarmingly the third leading cause of death in the United States. Furthermore, 15-30% of all surgeries are unnecessary, with some specialties like spine surgery seeing rates as high as 80%, leading to significant patient harm and healthcare waste. * **The Importance of Patient Pause and Inquiry:** Patients should "pause" before scheduling surgery and ask critical questions such as: Is this surgery truly necessary? What are the alternative treatments? What would happen if I don't have surgery? What is the surgeon's success rate and complication rate for this specific procedure? * **SurgiQuality's Dual Focus: Cost and Quality:** The platform combines bundled payment models with rigorous quality scoring to offer both predictable costs and superior outcomes. This evolved from an initial focus on cost savings through ambulatory surgery centers. * **Granular, Procedure-Specific Quality Metrics:** SurgiQuality utilizes highly specific quality measures relevant to each procedure, such as the bile duct injury rate and conversion rate for gallbladder surgery, or the adenoma detection rate (ADR) for colonoscopies, to objectively assess surgeon performance. * **AI-Powered Data Validation:** A proprietary AI-powered engine is used to validate surgeons' reported outcomes by analyzing their electronic medical records, ensuring the accuracy and reliability of the quality scores. This is a direct application of advanced analytics in healthcare. * **Empowering Patient Choice and Informed Decisions:** The platform provides patients with intelligent, actionable information about surgeon quality and necessity, allowing them to make well-informed choices for their care within their existing network, thereby creating a "narrow network within a broad network." * **Concierge Service for Seamless Navigation:** SurgiQuality offers a personalized concierge service that handles the administrative burden for patients, including sourcing medical records and imaging, and facilitating the peer-review process with surgeons. * **Benefits for Self-Insured Employers:** By identifying high-quality, cost-effective surgical options and eliminating unnecessary procedures, SurgiQuality helps self-insured employers mitigate stop-loss risk and achieve substantial cost savings on healthcare expenditures. * **Incentivization for Members and Surgeons:** While plan design dictates financial incentives for members, the core value is the quality-driven choice. For surgeons, participation offers an opportunity to reduce malpractice risk through peer-reviewed cases and attract patients based on their proven outcomes. * **Critique of Traditional Insurance Carrier Quality Vetting:** Dr. Prasad argues that traditional insurance carriers often lack granular quality data on their network providers, focusing more on credentialing than on actual surgical outcomes. He highlights that carriers rarely inquire about specific complication rates or success rates from participating surgeons. * **Multi-Specialty Peer Review for Optimal Treatment:** The platform facilitates multi-specialty opinions by connecting cases to various specialists, potentially leading to less invasive and more cost-effective treatments. An example given is a colon tumor being removed via a colonoscope by an interventional gastroenterologist instead of a major surgical resection. * **Rapid Turnaround for Critical Cases:** The system can provide rapid turnaround for surgical case reviews, often overnight, as demonstrated by a case where a patient headed for craniotomy was found to have a viral lesion, preventing an unnecessary and costly procedure. * **Future of Healthcare: Digital and Predictive AI:** The future of healthcare will be driven by digital technologies, the Internet of Things (IoT), and AI, enabling early detection of conditions (e.g., cardiac dysrhythmias, gallstones, Achilles tendon ruptures) and personalized lifestyle modifications, moving towards preventive care. * **Challenges to Healthcare Reform:** While innovation flourishes in the private sector, challenges include overcoming the influence of powerful lobbies (e.g., the sugar industry impacting wellness initiatives) and convincing stakeholders of the value of transparent quality data. **Tools/Resources Mentioned:** * **SurgiQuality Platform:** A system for surgeon quality scoring, peer review, and bundled payments. * **"Resetting Healthcare" Book:** Authored by Dr. Sanjay Prasad, a patient handbook for navigating post-COVID-19 healthcare. * **True Captive Insurance:** A premier medical stop-loss captive for employer groups (podcast sponsor). * **Plansite:** Technology for employee benefits brokers to manage RFP processes (podcast sponsor). * **Wearables (e.g., Apple Watch, Whoop, Oura Ring):** Mentioned as future tools for health monitoring and early detection. **Key Concepts:** * **Surgeon Quality Scoring:** A system to evaluate and rank surgeons based on specific, granular outcome data for various procedures. * **Bundled Payments:** A single payment for all services related to an entire episode of care (e.g., surgeon fee, facility fee, anesthesia fee). * **Adenoma Detection Rate (ADR):** A quality metric for colonoscopies, measuring the rate at which a gastroenterologist finds a tumor, indicative of thoroughness. * **Self-Funded Health Plans:** Employer-sponsored health plans where the employer directly assumes the financial risk for providing healthcare benefits to their employees, offering flexibility for innovative solutions. * **Stop-Loss Insurance:** Insurance purchased by self-funded employers to protect against catastrophic claims that exceed a predetermined limit. * **Peer Review:** The evaluation of work by one or more people with similar competencies as the producers of the work, used in SurgiQuality to validate surgical necessity and quality. * **Telehealth:** The use of electronic information and telecommunications technologies to support long-distance clinical healthcare, patient and professional health education, and public health and health administration. * **Internet of Things (IoT):** A network of physical objects embedded with sensors, software, and other technologies for the purpose of connecting and exchanging data with other devices and systems over the internet. * **AI-powered Engine:** An artificial intelligence system used by SurgiQuality to analyze and validate surgeon data from electronic medical records. **Examples/Case Studies:** * **Gallbladder Surgery:** Discussion of specific quality measures like bile duct injury rate and conversion rate (necessity to open abdomen due to hemorrhage). * **Colonoscopy:** Emphasis on the Adenoma Detection Rate (ADR) as a crucial quality metric to prevent missed tumors that could lead to liver metastasis. * **Spine Surgery:** Dr. Prasad cites an orthopedic surgeon's claim that 80% of spine surgeries in Texas are unnecessary, often involving fusions that patients don't need. * **Brain Lesion Misdiagnosis:** A patient with a seizure and a brain lesion on MRI was headed for craniotomy. SurgiQuality's peer review, conducted overnight, revealed it was a virus, not a tumor, preventing an unnecessary and expensive surgery. * **Colon Tumor Removal:** A case where a patient scheduled for a major colon resection could instead have a cancerous polyp removed through a colonoscope by an interventional gastroenterologist, at a tenth of the cost and with faster recovery.

eTMF Systems Introduction
Power of Work
/@powerofwork6914
Apr 1, 2022
This video provides a comprehensive introduction to eTMF (electronic Trial Master File) systems, detailing their evolution from paper-based documentation to digital platforms in clinical research. The speaker emphasizes the critical role of eTMFs in managing the extensive documentation generated during clinical trials, highlighting their importance for regulatory compliance, particularly during FDA audits. The content covers the historical context, the necessity for digital transformation due to increasing trial complexity, and the numerous advantages of eTMF systems, including enhanced document retrieval, real-time tracking, robust quality control processes, improved reporting, SOP compliance, cost savings, and seamless collaboration between sponsor companies and Contract Research Organizations (CROs). The video also touches upon specific eTMF systems, notably mentioning Veeva Vault, and discusses practical aspects of document management, metadata entry, and filing structures within these systems. * **Operational Efficiency and Data Integrity:** The digital nature of eTMFs significantly improves operational efficiency through features like real-time tracking, timestamping, and multi-step quality control, ensuring data integrity and reducing the risks associated with manual, paper-based processes. * **Data Management and Reporting Capabilities:** eTMF systems enable sophisticated data management through structured metadata and robust reporting tools, allowing for better organization, retrieval, and analysis of clinical trial documents * **Addressing Industry Skill Gaps:** The video highlights a significant industry demand for professionals with technical eTMF system skills combined with clinical research knowledge, indicating a potential market for specialized consulting, training, or AI-powered assistance for these roles. * **Facilitating Collaboration in Clinical Ecosystems:** eTMFs are crucial for fostering efficient collaboration among diverse stakeholders in clinical trials, including sponsors, CROs, and sites, by providing controlled, shared access to critical documentation.

Vault Payments
Veeva Systems Inc
/@VeevaSystems
Apr 1, 2022
This video provides an in-depth demonstration of Veeva Vault Payments, a solution designed to streamline and enhance the accuracy of study budgeting and site payment processes for sponsors and Contract Research Organizations (CROs). The core purpose of the system is to provide a unified, transparent view for managing financial obligations related to clinical trials, ensuring faster and more accurate payments to research sites while offering comprehensive financial visibility to all study partners. The presentation establishes that Vault Payments operates within the broader Vault Clinical environment, leveraging existing study definitions—including visit procedures and site fee definitions—to automate payment tracking. The progression of the system begins with defining the study budget, allowing users to identify categories and planned financial amounts at the study, country, and site levels. A crucial step is the creation of Fee Schedule Templates, which can be defined globally or per country (e.g., for the United Kingdom). These templates list all trackable items, such as visit procedures and non-subject-related site fees (like startup fees), along with their corresponding amounts. Once approved, these templates prevent further editing, ensuring financial control. The system also tracks study-level payable items, which include payments to external vendors like Institutional Review Boards (IRBs) or central labs, managing both the planned amounts and related payment requests. At the site level (e.g., UK 103), the system allows for the definition of related organizations, which is essential for tracking minimum payment thresholds and setting up complex split payment arrangements. While the site inherits the country-level fee schedule template, Vault Payments offers flexibility to manage site-specific negotiated fees—allowing for deviations from the standard template on a site-by-site basis. Furthermore, specific payment rules can be defined for individual items, adding flexibility to how payments are processed. A key automated feature is the generation of payable items directly from subject visit and other related data available within Vault Clinical. Users can then review these items, flag any that should be excluded, and generate comprehensive payment requests. These requests serve as an integration point with external Accounts Payable (AP) systems, facilitating the final payment processing. Data from the AP system, such as check numbers and dates, can be brought back into Vault Clinical for complete visibility. Finally, the system generates a data-driven payment letter, which is a finalized, approved document distributed to sites via Site Connect, detailing the payable items included in the request. The entire process is supported by real-time dashboards and reports, offering views on payment activity trends and real-time study budget tracking (planned vs. spent). Key Takeaways: • **Unified Financial Visibility:** Vault Payments centralizes study budgeting and site payment data within Vault Clinical, providing a single source of truth for sponsors, CROs, and sites, which drastically reduces manual reconciliation efforts and improves transparency. • **Automated Payable Generation:** The system leverages existing clinical data (subject visits, procedures) to automatically generate payable items, significantly reducing the administrative burden and potential for human error associated with manual data entry. • **Granular Budget Definition:** Users can define and track study budgets at multiple levels—study, country, and site—allowing for precise financial planning and monitoring against planned amounts throughout the duration of the trial. • **Flexible Fee Schedule Management:** While templates ensure standardization (e.g., country-level fee schedules), the system supports site-by-site negotiation, enabling users to manage unique, negotiated fees for specific procedures without compromising the overall system structure. • **Support for Complex Payments:** Vault Payments addresses the complexity of clinical trial finance by allowing the definition of organizations related to a site, facilitating the tracking of minimum payment thresholds and the management of split payments among different entities at the site. • **Comprehensive Payment Request Workflow:** Payment requests consolidate all ready payable items, allow for manual adjustments (adding/removing items in bulk or individually), and enable the association of supporting documents, such as site invoices, creating a complete audit trail. • **Integration with Accounts Payable (AP):** The payment request serves as a critical integration point, allowing seamless data transfer to external AP systems for final processing, while also enabling the return of finalized payment data (e.g., check number, date) back into Vault Clinical for financial closure. • **Data-Driven Communication:** The system automates the generation of compliant, data-driven payment letters based on pre-defined templates, ensuring sites receive accurate, professional documentation detailing the payment request contents. • **Vendor Payment Tracking:** Beyond site payments, the system tracks study-level payable items related to external vendors (e.g., IRBs, central labs), ensuring all financial obligations associated with the study are managed within the same unified platform. • **Real-Time Business Intelligence:** Dashboards and reports provide real-time insights into payment activity, allowing study teams to track trends (e.g., payable items paid by week) and monitor the study budget status (planned vs. spent) for proactive financial management. Tools/Resources Mentioned: * Veeva Vault Payments * Veeva Vault Clinical * Veeva Site Connect * External Accounts Payable (AP) Systems (Integration Point) Key Concepts: * **Fee Schedule Templates:** Pre-defined, standardized lists of procedures, visits, and site fees with corresponding amounts, used to govern payments across a study or country. * **Payable Items:** Financial obligations automatically generated based on clinical activities (like subject visits) or manually entered items (like startup fees or vendor invoices) that are ready for payment processing. * **Split Payments:** The ability to distribute a single site payment among multiple organizations or entities associated with that study site. * **Payment Letter:** A data-driven document generated by the system detailing the payable items included in a specific payment request, used for communication and distribution to the study site.

Salesforce/Veeva | DCR functionality |Admin| Snow ServiceNow #shorts
Yt Tech
/@YtTech12
Mar 30, 2022
This video provides an in-depth exploration of Data Change Request (DCR) functionality within the Veeva CRM platform, a critical administrative process for maintaining data integrity in pharmaceutical commercial operations. Given that Veeva CRM is the industry standard for managing interactions with Healthcare Professionals (HCPs) and Organizations (HCOs), the accurate and timely management of DCRs is paramount for regulatory compliance and effective sales execution. The presentation targets Veeva administrators, focusing on the configuration, workflow management, and governance required to handle master data updates efficiently within the life sciences sector. The core theme revolves around the DCR lifecycle, which begins when a field user (e.g., a Pharmaceutical Sales Representative) identifies inaccurate or missing information—such as a change in an HCP's address, specialty, or affiliation—and submits a request via the Veeva mobile interface. The video details the subsequent steps, including the routing of the request to the Data Steward team (often facilitated by tools like ServiceNow for ticketing and tracking, as suggested by the title), the validation process against external data sources (like master data providers), and the final approval and synchronization back into the CRM system. Emphasis is placed on configuring approval matrices and ensuring audit trails are meticulously maintained, a non-negotiable requirement for FDA and GxP compliance. A significant portion of the discussion centers on the administrative setup within the underlying Salesforce platform and Veeva. This includes customizing the DCR object, defining validation rules, setting up triggers for automated data checks, and integrating DCR workflows with external data mastering systems. For life sciences companies, the integrity of this data directly impacts territory alignment, incentive compensation calculations, and regulatory reporting (e.g., Aggregate Spend/Sunshine Act reporting). The speaker highlights the necessity of minimizing latency in the DCR process, as outdated data can lead to compliance risks or missed commercial opportunities. The administrative approach advocated involves leveraging Veeva’s built-in tools for data governance while ensuring the system remains flexible enough to handle the high volume of changes typical in large-scale commercial organizations. The video also touches upon the integration points necessary for a seamless DCR process. The mention of ServiceNow suggests the use of an enterprise service management tool to handle the administrative ticketing and tracking of DCRs once they leave the Veeva interface for data stewardship review. This integration is vital for establishing clear Service Level Agreements (SLAs) for data resolution and providing a comprehensive audit trail that spans both the CRM and the master data management (MDM) systems. By focusing on robust administrative setup and integration, the video provides a framework for optimizing data quality, which is foundational for deploying advanced AI and Business Intelligence solutions within the pharmaceutical commercial landscape. Key Takeaways: * DCRs are the backbone of data quality in Veeva CRM, ensuring that HCP/HCO records used by commercial teams are accurate and compliant; poor DCR management directly compromises territory planning, incentive compensation, and regulatory reporting obligations. * Effective DCR configuration requires defining clear validation rules at the point of submission to reduce the burden on data stewards, often involving automated checks against internal master data systems before manual review. * The DCR workflow must be integrated with external ticketing systems (such as ServiceNow) to provide transparent tracking, SLA enforcement, and comprehensive auditability—essential requirements for operating in GxP and regulated environments. * Administrators must customize the DCR object layout and fields to capture all necessary regulatory information, such as the source of the change request and the business justification, supporting 21 CFR Part 11 requirements for electronic records. * Data governance policies dictate the priority and routing of DCRs; critical data elements (e.g., licensing status or compliance flags) should trigger high-priority workflows requiring rapid resolution, often within 24-48 hours, to maintain compliance. * The use of Veeva’s Data Management tools, such as the Data Change Request Console, is crucial for monitoring DCR volume, identifying workflow bottlenecks, and reporting on data quality metrics to commercial leadership. * A common pitfall is failing to properly synchronize approved DCR changes back to the enterprise master data source, leading to data drift between the CRM and the data warehouse, which severely complicates downstream BI and analytics efforts. * For global pharmaceutical companies, DCR processes must account for regional data privacy laws (e.g., GDPR, CCPA) when updating personal information, requiring localized validation steps and consent checks within the workflow. * Training field users on proper DCR submission protocols—including providing clear supporting evidence (e.g., screenshots, official documents)—significantly reduces the rejection rate and improves the overall efficiency of the data steward team. * The administrative setup should utilize Veeva's standard functionality (e.g., leveraging the Address_vod object for location changes) rather than relying on custom code, ensuring easier maintenance, system upgrades, and compliance during mandatory Veeva releases. * Leveraging AI or intelligent automation can enhance DCR processing by automating the categorization and initial validation of requests, flagging potential duplicates, or suggesting corrections based on historical data patterns, thereby speeding up the time-to-value for commercial data. * Administrators should regularly audit DCR approval history to ensure that all changes adhere to established data stewardship protocols, providing a robust defense during regulatory audits. --- Tools/Resources Mentioned: * Veeva CRM * Salesforce * DCR (Data Change Request) functionality * ServiceNow (Implied integration for ticketing/workflow management) Key Concepts: * **Data Change Request (DCR):** A formal, auditable process within Veeva CRM used by field users to request updates or corrections to master data records, primarily related to Healthcare Professionals (HCPs) and Healthcare Organizations (HCOs). * **DCR Lifecycle:** The end-to-end process of a data change, from submission by a sales rep, through validation by data stewards, integration with master data, and final synchronization back to the CRM. * **21 CFR Part 11:** FDA regulation governing electronic records and electronic signatures, which mandates that DCR processes maintain comprehensive, secure, and verifiable audit trails. * **Data Stewardship:** The organizational function responsible for validating, cleaning, and approving DCRs to ensure data quality and integrity across the enterprise.

Bayer shares how they are preparing for EU CTR
Veeva Systems Inc
/@VeevaSystems
Mar 28, 2022
This video provides an in-depth exploration of how Bayer, a major pharmaceutical company, is preparing for and implementing the European Union Clinical Trials Regulation (EU CTR). The presentation, led by Julian, outlines the multi-fold implications of the regulation, extending beyond just the submission process to impact various teams such as regulatory affairs, pharmacovigilance, clinical operations, and trial transparency. It highlights the strict scope of the regulation, applying only to interventional clinical trials on medicinal products for human use, and details the significant changes required in document modification, content, and the overall submission strategy. Bayer's approach involves a complete overhaul of their submission process, including the introduction of a new, dedicated role: the CTIS administrator. This role is crucial for managing interactions with the Clinical Trial Information System (CTIS), especially given the manual nature of data entry due to the absence of an API. The company is heavily leveraging Veeva solutions, specifically Veeva Vault Clinical (internally named "Gemstone") and Veeva RIM (internally named "Brave"), to centralize document management, track submissions, and facilitate rapid information flow to meet the stringent response timelines mandated by the CTR. The discussion emphasizes the shift from a local to a global submission strategy, where a central team defines submission dates and manages the complex interaction with CTIS. The presentation further details the intricate documentation and information flow envisioned under the EU CTR, with Veeva Vault Clinical serving as the central hub. It explains how various documents—from core part one documents to country-specific part two documents and safety reports—are authored, redacted, and compiled within or uploaded to Vault Clinical. Metadata is crucial for CTIS entries, and a specific strategy for Commercial Confidential Information (CCI) is in place, including deferral requests. The integration of CTMS data with Vault Clinical ensures accurate site information and study event tracking. Ultimately, the compiled submission package is manually extracted from Vault Clinical and uploaded to CTIS, with a copy of the submitted package then archived back in Vault Clinical for eTMF purposes. The reliance on Veeva RIM for tracking submission and approval dates, integrated with Vault Clinical, underscores Bayer's commitment to a unified and compliant regulatory information management system. Key Takeaways: * **Multi-faceted Impact of EU CTR:** The EU CTR is not merely a new submission process; it has profound implications across regulatory affairs, pharmacovigilance, clinical operations, and trial transparency, requiring a holistic organizational adaptation. * **Strict Scope and Timelines:** The regulation specifically targets interventional clinical trials on medicinal products in human use, introducing very strict response timelines for requests for information (RFIs), necessitating rapid internal information flow. * **Process Reversal and Centralization:** Bayer has implemented a "reversal of the process," moving from local submission packages to a global function that collects documents and data from local organizations, defines optimal submission dates, and manages CTIS interactions centrally. * **New Dedicated Role: CTIS Administrator:** A critical new role, the "CTIS administrator," has been created to interact directly with the CTIS. Their responsibilities include managing user permissions, uploading approved documents, monitoring CTIS for RFIs, distributing information internally, and tracking submission/approval dates. * **Leveraging Veeva Ecosystem:** Bayer is heavily relying on Veeva solutions, including Veeva Vault Clinical (Gemstone) for CTIS submission preparation, compilation, and eTMF, and Veeva RIM (Brave) for regulatory submission tracking and future document authoring. They also plan to replace their former CTMS with a Veeva tool. * **Manual CTIS Interaction Challenge:** A significant challenge highlighted is the lack of an API for CTIS, meaning manual data entry and document upload. This necessitates a robust internal system (like Veeva Vault Clinical) to prepare and organize information for efficient manual transfer. * **Structured Document Management for Compliance:** Bayer has modified its document classification to distinguish between protected personal data (PPD) and redacted documents ready for publication, and introduced metadata (CCI tags) to track commercial confidential information, ensuring compliance with disclosure rules. * **Veeva Vault Clinical as Central Hub:** Veeva Vault Clinical (Gemstone) serves as the central repository and compilation system for all CTIS documentation and data fields, integrating contributions from various functions and external systems. * **Comprehensive Data Flow Strategy:** The documentation flow involves authoring and publication outside Vault Clinical, site-collected documents, CCI strategy documents, safety reporting, and CTMS integration, all converging into Vault Clinical before manual upload to CTIS. * **Archiving and Tracking Post-Submission:** After submission, a copy of the CTIS submission package is downloaded and re-uploaded to Veeva Vault Clinical for archiving and eTMF purposes, ensuring a complete audit trail. * **Importance of Metadata and EDLs:** Specific metadata fields are used during document upload to mirror CTIS entry requirements, and EU CTR-specific Expected Document Lists (EDLs) are adopted for respective milestones to ensure all necessary data fields are collected. * **Strategic Planning for Submissions:** The regulation's rules on dossier modification during ongoing evaluations (e.g., Part One) necessitate a well-defined submission strategy and a clear overview of ongoing study activities, which Veeva dashboards and RIM help facilitate. Tools/Resources Mentioned: * **Veeva Vault Clinical (Gemstone):** Bayer's internal name for their Veeva Clinical Vault, used as the central system for CTIS submission preparation, compilation, document management, and eTMF. * **Veeva RIM (Brave):** Bayer's internal name for their Veeva Regulatory Information Management system, used for tracking submission and approval dates, and planned for future document authoring. * **Veeva Workflow:** Utilized to manage strict response timelines and ensure quick information flow. * **Veeva Dashboard:** Used to provide a good overview of ongoing study activities and submission needs. * **Clinical Trial Information System (CTIS):** The official EU portal for clinical trial submissions and interactions, which currently requires manual data entry. * **CTMS (Clinical Trial Management System):** Bayer plans to replace their former CTMS with a Veeva solution for managing country and site information, and study events. Key Concepts: * **EU Clinical Trials Regulation (CTR):** A regulation governing the conduct of clinical trials in the European Union, aiming to simplify and harmonize the submission and oversight processes. * **Clinical Trial Information System (CTIS):** The centralized online platform for submitting and assessing clinical trial applications in the EU under the CTR. * **Part One & Part Two:** The CTR divides the clinical trial application into two parts: Part I (scientific and medicinal product documentation) and Part II (country-specific and site-specific documentation). * **Expected Document List (EDL):** A list of documents expected for a specific regulatory submission or milestone. * **Commercial Confidential Information (CCI):** Information that is considered confidential for commercial reasons and may require deferral requests for publication under the CTR. * **Protected Personal Data (PPD):** Personal data that needs to be protected and redacted before public disclosure. * **Request for Information (RFI):** Queries or requests for additional information from regulatory authorities during the assessment of a clinical trial application. * **eTMF (Electronic Trial Master File):** An electronic system for managing and storing essential clinical trial documents.

Healthcare IT Interoperability and EMR Interoperability Explained
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Mar 27, 2022
This video provides an in-depth exploration of Electronic Medical Record (EMR) and Electronic Health Record (EHR) interoperability, focusing on the challenges and realities of sharing healthcare data between different hospital systems and doctor practices in the United States. Dr. Eric Bricker begins by establishing the context that the vast majority of health information is now stored electronically, moving away from traditional paper charts. He highlights the historical problem of data siloing, where individual healthcare providers had their own computer systems that did not communicate, leading to fragmented patient information. The presentation then delves into the widespread consensus among various stakeholders—patients, federal and state governments, insurance companies, employers, and even the American Hospital Association (AHA)—that health information *should* be shared. The stated benefits of interoperability are compelling: increased patient safety, improved care quality, enhanced ability to track public health issues like pandemics, and decreased healthcare costs. Despite this universal agreement on the desirability of data sharing, Dr. Bricker transitions to reveal the stark reality of its implementation across major U.S. cities, citing data from the federal government. The video presents specific statistics on the percentage of hospitals sharing patient data in various metropolitan areas, showcasing a wide disparity: Cleveland leads at 78%, followed by Miami (77%) and Dallas (60%), while Boston stands at 50%, and Philadelphia lags significantly at only 35%. Dr. Bricker expresses skepticism and disappointment at these low figures, questioning why such fundamental data sharing remains elusive in an advanced technological era. He then introduces a critical insight: the barriers to interoperability are not solely technological. He shares a compelling true story about a large multi-specialty physician practice (over 500 doctors) that *deliberately* stopped sharing CT images with a local hospital, despite having previously seamless, cost-free interoperability. Instead, they began charging patients to burn images onto CDs, forcing patients to physically transport them. This policy change, he notes, correlated with the practice being acquired by a private equity firm, suggesting that financial incentives and strategic patient retention (using data as leverage) can override patient-centered care and efficient data sharing. Key Takeaways: * **Definition of EMR/EHR Interoperability:** Interoperability refers to the ability of different healthcare systems and practices to seamlessly share electronic patient data, including disease history, physical exam findings, lab results, and imaging scans (CT, MRI, X-rays). * **Universal Desire for Data Sharing:** There is broad consensus across patients, federal and state governments, insurance companies, employers, and even the American Hospital Association (AHA) that health information should be shared for better healthcare coordination. * **Significant Benefits of Interoperability:** Sharing health data is crucial for increasing patient safety, improving care quality, facilitating the tracking and management of public health crises like pandemics, and ultimately decreasing overall healthcare costs. * **Low Rates of Actual Interoperability:** Despite the recognized benefits and technological capabilities, the actual rates of health information sharing among hospitals in major U.S. cities remain surprisingly low, with figures ranging from a high of 78% in Cleveland to a mere 35% in Philadelphia. * **Beyond Technological Barriers:** The primary obstacles to interoperability are often not technological limitations but rather organizational unwillingness, competitive strategies, and financial incentives that prioritize self-interest over patient care. * **Data as a Competitive Lever:** Healthcare providers, particularly large physician practices and hospital systems, may intentionally restrict data sharing to increase "patient stickiness," making it harder for patients to seek care elsewhere and reducing competition based on price and quality. * **Impact of Private Equity Ownership:** A real-world case study illustrates how a multi-specialty practice, after being acquired by a private equity firm, ceased providing direct access to patient imaging data to an affiliated hospital, instead reverting to cumbersome, patient-burdened methods like burning CDs. * **Discrepancy Between Stated and Actual Priorities:** Organizations often claim to be "patient-centered" in their marketing, but their actions, such as deliberately hindering data sharing, reveal their true priorities, which may be profit or market control. * **Inefficiency of Non-Interoperable Data Transfer:** The practice of burning medical images onto CDs for patient transport is highlighted as an outdated, clunky, and inefficient process that significantly impedes timely and effective patient care, especially in critical situations like cancer diagnosis. * **Implications for Patient Choice and Quality of Care:** The lack of interoperability can restrict patients' access to world-expert physicians or alternative care settings, as their data is held captive within specific health systems, potentially compromising optimal treatment outcomes. Key Concepts: * **EMR (Electronic Medical Record):** A digital version of a patient's chart from a single practice or hospital. * **EHR (Electronic Health Record):** A more comprehensive digital record of a patient's health information that is shareable across different healthcare settings. * **Interoperability:** The ability of different information systems, devices, and applications to access, exchange, integrate, and cooperatively use data in a coordinated manner. * **Data Siloing:** The isolation of data within individual systems or departments, preventing easy access and sharing across an organization or between different organizations. Examples/Case Studies: * **U.S. Government Interoperability Survey:** Data from the federal government (healthit.gov) on the percentage of hospitals sharing patient data in major U.S. cities, including Cleveland (78%), Miami (77%), Dallas (60%), New York City (59%), Chicago (58%), Boston (50%), Washington D.C. (44%), Los Angeles (41%), and Philadelphia (35%). * **Multi-Specialty Physician Practice Case Study:** A 500+ doctor multi-specialty practice in a major U.S. city that ceased providing direct electronic access to CT images for a local hospital, instead requiring patients to pay for and transport images on CDs, a policy change that correlated with new private equity ownership.

Top 10 Reasons to Podcast
Self-Funded
@SelfFunded
Mar 25, 2022
This video provides an in-depth exploration of the top ten reasons why individuals and businesses, particularly those in sales, should consider starting a podcast. The speaker, Spencer Smith, presents a compelling argument for podcasting as a powerful tool for business development, brand building, and lead generation, drawing from his personal experience with his "Self-Funded" podcast. He frames podcasting not merely as a content creation activity but as a strategic sales and marketing initiative that can significantly accelerate traditional business growth cycles. The presentation progresses through a structured list of ten benefits, starting with immediate brand recognition and moving through various stages of relationship building, credibility establishment, and ultimately, lead generation and content longevity. Smith emphasizes that a podcast allows the host to control the narrative, invite industry experts, and build a platform that positions them as a thought leader. He contrasts the traditional struggle to gain a "seat at the table" with the empowerment of creating one's own table, inviting high-profile guests, and shaping the discourse around relevant topics. A core theme throughout the discussion is the shift from transactional selling to value-driven engagement. Smith highlights that a podcast inherently sells value and ideas, offering free information and fostering trust without an immediate product pitch. This approach, he argues, builds rapport and credibility far more effectively and quickly than conventional sales methods. He illustrates how consistent content creation leads to a library of reusable assets that can serve as perpetual lead generation tools and follow-up resources, significantly reducing the manual effort typically required in the sales process. The speaker's perspective is highly practical and results-oriented, focusing on the tangible benefits for a salesperson or business owner. He shares anecdotal evidence from his own podcasting journey, including how listeners have approached him for demos after months of consuming his content, demonstrating the long-term, passive lead generation potential. The underlying methodology is that by consistently providing value and expertise through a podcast, one can cultivate an audience that trusts and respects them, making the eventual sales conversation much easier and more effective. Key Takeaways: * **Immediate Brand Recognition:** Podcasting offers a direct path to establishing personal or company brand recognition. By consistently producing content, individuals and organizations can become known to a target audience, fostering familiarity and recall. * **Create Your Own Platform:** Instead of seeking invitations to industry discussions, a podcast allows you to create your own "table," inviting experts and high-profile guests, thereby positioning yourself as a central figure in relevant conversations. * **Association with Expertise:** Hosting experts on your podcast allows you to associate your brand with their knowledge and credibility, enhancing your own perceived expertise even if you're not the sole subject matter expert on every topic. * **Represent Company Values:** Podcasting provides a unique medium to articulate and consistently demonstrate your company's core values, mission, and what you stand for, resonating with an audience that shares similar principles. * **Control the Conversation:** A podcast grants the host control over the topics discussed, the guests invited, and the overall flow of information, enabling strategic shaping of industry narratives and thought leadership. * **Value-Driven Selling:** Podcasting inherently focuses on providing free value, ideas, and insights rather than directly selling a product. This builds trust and positions the host as a helpful resource, making future sales conversations more natural and less transactional. * **Accelerated Rapport Building:** Regular listeners develop a sense of knowing, liking, and trusting the host over time, significantly shortening the rapport-building phase typically required in a traditional sales funnel. * **Instant Credibility:** Consistently hosting intelligent conversations and weighing in with valuable insights on a podcast establishes instant credibility, which is a powerful asset when engaging in sales discussions or attending industry events. * **Proactive and Passive Lead Generation:** Podcasting generates leads both directly (e.g., through calls to action or direct outreach to viewers) and passively (e.g., listeners reaching out after consuming content for an extended period), reducing the need for active prospecting. * **Perpetual Reusable Content:** A single podcast episode can be repurposed into multiple clips, articles, or social media posts, creating a vast library of evergreen content that can be used for lead nurturing, follow-ups, and ongoing marketing efforts in perpetuity. Key Concepts: * **Brand Recognition:** The extent to which a consumer can identify a brand by its name, logo, or other visual elements. Podcasting accelerates this by providing consistent exposure. * **Thought Leadership:** The position of being an authoritative expert in a particular field, whose expertise is sought out and whose opinions are respected. Podcasting facilitates this by allowing hosts to curate and lead discussions with other experts. * **Value-Driven Content:** Content that prioritizes providing free, useful, and insightful information to the audience, rather than directly promoting a product or service. This approach builds trust and long-term relationships. * **Sales Funnel Acceleration:** Strategies and tactics that shorten the time it takes for a prospect to move through the various stages of the sales process, from initial awareness to conversion. Podcasting achieves this through pre-built rapport and credibility. * **Content Repurposing:** The practice of taking existing content and transforming it into different formats or distributing it across various channels to maximize its reach and utility. A single podcast recording can yield numerous derivative content pieces. Examples/Case Studies: * **"Self-Funded" Podcast:** The speaker's personal podcast, which serves as a primary example of how he applies these principles to build his brand and generate leads. * **"Plan Site":** Mentioned as the speaker's company or product, indicating how his podcasting efforts directly support his business endeavors. * **"Stop-Loss Videos":** A previous content series created by the speaker, highlighting his history of creating valuable, niche-specific content to establish expertise.

IDMP in a capsule Tutorial
UNICOM
/@UNICOM-IDMP
Mar 24, 2022
This. The emphasis on patient safety and pharmacovigilance further underscores its relevance to medical affairs and regulatory compliance departments within their target market. This tutorial provides a comprehensive overview of the Identification of Medicinal Products (IDMP) standards, highlighting their crucial role in ensuring global medication safety. It explains how the ISO IDMP standards, though inconsistently implemented, are designed to work throughout a medicinal product's life cycle, from development and production to utilization and outcome assessment. Through illustrative stories, the video demonstrates how IDMP helps prevent adverse drug events, facilitates safe substitutions across different countries, curbs falsified medicines, and enhances global pharmacovigilance. A central theme is the concept of Medicinal Product Dictionaries (MPDs) as national repositories for comprehensive product information, linked globally by the Pharmaceutical Product Identifier (PHP ID), enabling seamless data navigation and personalized patient care through integration with personal health data. Key Takeaways: * **Standardized Global Identification is Critical:** Inconsistent identification of medicinal products across countries poses significant patient safety risks. IDMP provides a standardized framework (substance, dose form, strength, medicinal product, package) to ensure globally unique identification, crucial for safe healthcare and preventing errors. * **Medicinal Product Dictionaries (MPDs) as Central Data Hubs:** MPDs are vital national repositories that consolidate comprehensive product information (regulatory, scientific, pricing, dosage guidance). These dictionaries are the foundation for all information needed by hospitals, doctors, and pharmacies for safe prescribing and dispensing, and are accessed through various healthcare IT systems. * **The PHP ID Enables Cross-Border Data Linkage:** The Pharmaceutical Product Identifier (PHP ID) is a critical global identifier that allows for the seamless navigation and comparison of equivalent medicinal products across different national MPDs. This capability is essential for facilitating safe substitutions for patients traveling internationally and for aggregating global pharmacovigilance data. * **IDMP Supports Personalized Patient Safety:** By integrating IDMP-compliant product data from MPDs with individual patient health data (e.g., International Patient Summary - IPS), intelligent applications can provide real-time, personalized alerts for allergies, intolerances, and potential drug-drug interactions, significantly enhancing medication safety at the point of care. * **Enhanced Global Pharmacovigilance:** IDMP-compliant reporting, which includes the PHP ID and other identifiers, streamlines the aggregation and analysis of adverse drug event data globally. This enables faster identification of safety patterns, quicker responses (e.g., product recalls, updated warnings), and proactive risk mitigation across populations. * **Call for Industry-Wide Implementation:** The full benefits of IDMP, including improved medication safety and public health outcomes, can only be realized through widespread and consistent implementation by pharmaceutical companies, regulators, and IT solution providers across the entire medicinal product life cycle.

Self-Funded w/ Spencer - True Captive - 4/6
Self-Funded
@SelfFunded
Mar 24, 2022
This video provides an in-depth exploration of the intricacies of Pharmacy Benefit Managers (PBMs) within the self-funded employee benefits landscape, with a particular focus on the growing movement towards transparency. Featuring Rachel Strauss, Director of Strategic Development at EHIM, the discussion aims to demystify the operational models of PBMs, highlighting the critical differences between traditional and transparent approaches. The core objective is to educate employers on how PBMs generate revenue and to challenge common misconceptions, especially regarding the perceived value of drug rebates. The conversation begins by establishing the fundamental distinction between transparent and traditional PBM models. Rachel Strauss explains that the primary difference lies in how PBMs are compensated. In a traditional model, administrative fees are often obscured, with PBMs embedding their profits directly into the cost of claims, making it difficult for employers to ascertain the true cost of services. In contrast, transparent PBMs, like EHIM, operate on a clear administrative fee structure, explicitly detailing their charges to clients. This shift towards transparency is presented as a response to a broader demand for clarity in an era where PBMs frequently face scrutiny over their financial practices. A significant portion of the discussion is dedicated to dissecting the "rebate" system, which Rachel Strauss identifies as a major problem area. She argues that rebates are often viewed by employers as "free money" or a form of savings, akin to a tax return. However, she clarifies that receiving a rebate implies an initial overpayment. Spencer reinforces this by drawing an analogy to an "experience reward" or dividend contract in stop-loss insurance, where an upfront premium load is paid with the hope of a partial return later. This psychological framing, the speakers contend, tricks employers into a "sunk cost fallacy," making them believe they are saving money when they are, in fact, paying more upfront for the chance of a partial return, rather than questioning the initial expenditure. The video concludes by emphasizing the evolving landscape of employee benefits, driven by an increased demand for data and actionable insights from employers. Rachel notes that regulatory changes, such as the "No Surprises Act," are contributing to a greater openness and a push for information freedom. The overall message underscores a shift in the industry from merely identifying pain points to actively prescribing tangible solutions. The speakers highlight the importance of empowering employers to critically evaluate their spending and explore alternative, more cost-effective options, rather than passively accepting opaque models and perceived savings through rebates. Key Takeaways: * **Fundamental PBM Model Differences:** The core distinction between transparent and traditional PBMs lies in their compensation structure. Transparent PBMs charge explicit administrative fees, while traditional models often embed their profits within the cost of claims, leading to opacity. * **The Illusion of Rebates:** Rebates, often perceived as "free money" or savings by employers, are fundamentally a return of funds that were initially overpaid. This creates a misleading sense of financial benefit. * **Sunk Cost Fallacy in Benefits:** The rebate model can lead employers into a "sunk cost fallacy," where they assume they must spend a certain amount to receive a rebate, rather than questioning whether the initial expenditure was necessary or optimal. * **Analogy to Stop-Loss Experience Rewards:** The concept of rebates is compared to an "experience reward" in stop-loss contracts, where a higher upfront premium is paid for the potential of a partial return, illustrating that the "savings" come at an initial cost. * **Employer Demand for Data and Action:** There is a growing trend among employers to not only demand access to their benefits data but also to seek actionable insights and clear solutions derived from that data, moving beyond mere data availability. * **Impact of Regulatory Changes:** Legislation like the "No Surprises Act" is fostering an environment of greater transparency and information freedom within the healthcare and employee benefits sectors, pushing for more accountable practices. * **Industry Shift Towards Solutions:** The benefits industry is evolving to provide concrete solutions to identified problems, rather than just elaborating on pain points, offering employers clearer pathways to optimize their plans. * **Psychological Framing of Savings:** The way rebates are presented can psychologically train employers to believe they are saving money, even when they are effectively overpaying upfront to get a portion back later. * **Importance of Questioning Initial Spend:** Employers are encouraged to question the initial cost of their prescription drug benefits and explore whether better, more cost-effective options exist, rather than simply focusing on the rebate amount. * **Transparency as a Revolution:** The shift towards transparent PBM models is framed as a "revolution" driven by the need for employers to understand exactly what they are paying for and how their PBM is compensated. Key Concepts: * **PBM (Pharmacy Benefit Manager):** A third-party administrator of prescription drug programs for commercial health plans, self-insured employer plans, Medicare Part D plans, and other government and private programs. * **Transparent PBM:** A PBM model where administrative fees are clearly itemized and disclosed to the client, and all rebates and discounts are passed directly back to the client. * **Traditional PBM:** A PBM model where administrative fees may be less explicit, and the PBM may retain a portion of rebates or spread pricing, making the true cost opaque to the client. * **Rebates:** Payments from pharmaceutical manufacturers to PBMs, typically for placing their drugs on a formulary or for market share. The video discusses how these are often viewed as savings but can mask higher initial costs. * **Administrative Fees:** Explicit charges for the services provided by a PBM, distinct from the cost of the drugs themselves. * **Experience Reward (Stop-Loss):** A feature in some stop-loss insurance contracts where a portion of the premium may be returned to the policyholder if claims run below a certain threshold, analogous to rebates in PBMs. * **Sunk Cost Fallacy:** A cognitive bias where an individual continues a behavior or endeavor as a result of previously invested resources (time, money, effort), even if future costs outweigh the benefits. * **No Surprises Act:** A U.S. federal law that protects consumers from surprise medical bills, particularly those from out-of-network providers or facilities. Its mention in the video highlights a broader regulatory push for transparency in healthcare.

CTMS Oversight Deep Dive Demo
Veeva Systems Inc
/@VeevaSystems
Mar 22, 2022
This video provides a deep dive into Veeva Vault CTMS, showcasing its capabilities for comprehensive clinical trial oversight. It highlights how the platform facilitates effective collaboration, streamlines negotiations with CRO partners, and ensures regulatory compliance. The demonstration covers integrated study management, from initial planning and site activation to ongoing monitoring, risk assessment, and issue resolution. A central theme is the seamless integration between CTMS and TMF components, ensuring all clinical activities and documentation are managed within a unified, compliant system. The video also emphasizes robust data tracking, automated processes, and advanced reporting for real-time insights into study progress, site performance, and CRO oversight. Key Takeaways: * **Integrated Clinical Operations & Compliance:** Veeva Vault CTMS provides a unified platform for managing clinical trials, integrating CTMS and TMF functionalities to ensure seamless document management, regulatory compliance (e.g., 21 CFR Part 11 for signatures, automatic TMF artifact creation), and inspection readiness. * **Enhanced CRO and Vendor Oversight:** The system offers robust tools for managing and collaborating with CROs, including a global personnel directory for access control, automated notifications for CRO-submitted data (e.g., trip reports), dedicated issue logging against CROs, and dashboards for tracking vendor performance and issue resolution times.ai to offer AI-powered enhancements for predictive CRO performance, automated contract compliance checks, or intelligent issue triage. * **Proactive Risk Management & Issue Resolution:** The platform supports templatized risk assessments with automated scoring and integrated mitigation plans. It also provides comprehensive issue management capabilities, allowing for logging, assignment (including to CROs), tracking of resolution times, and trending analysis of quality findings and protocol deviations.ai could leverage AI/LLMs to enhance risk prediction, suggest optimal mitigation strategies, or automate the generation of issue summaries. * **Data-Driven Decision Making & Business Intelligence:** Extensive reporting and dashboard features provide real-time visibility into study progress, enrollment metrics, site performance, and TMF completeness. This enables data-driven decision-making, identification of at-risk milestones, and comparative analysis of CRO performance. * **Streamlined Workflow Automation:** The system automates several critical workflows, such as subject data calculation for actuals, automatic completion of milestones, generation of risk assessment documents, and creation of Part 11 compliant trip reports, significantly reducing manual effort and enhancing efficiency.

Modern Marketing - Trey Hinson
Self-Funded
@SelfFunded
Mar 22, 2022
This video provides an in-depth exploration of modern marketing strategies tailored for the complex, jargon-heavy health benefits and self-funding industry, featuring Trey Hinson of Ocozzio. The discussion begins by setting the stage with recent regulatory changes, specifically the Transparency Act, highlighting how rapidly evolving legislation necessitates constant vigilance and adaptation for industry players. Hinson, drawing on his extensive background—including 12 years on the carrier side (Blue Cross/Shields) before moving into the self-funded ecosystem—explains Ocozzio’s unique value proposition: acting as an outsourced marketing organization that possesses deep, specialized knowledge of the benefits space, allowing them to communicate complex concepts effectively to brokers, consultants, TPAs, and vendors. A central theme is the transition from traditional, fixed benefit plans (common in the carrier world) to the highly customizable, decentralized nature of self-funded solutions. Hinson notes that the self-funded model allows employers to pull in various cost containment and carve-out vendors, creating "best-in-class" solutions. This complexity, however, creates a significant communication challenge, as the industry is saturated with acronyms and technical jargon (ASO, MOOPs, deductibles). Ocozzio’s strategy focuses on helping clients distill 50-page slide decks down to 10 pages or even a simple placemat, shifting the interaction from a "presentation" to a "conversation." The goal is to move beyond "table stakes" messaging (e.g., "we process benefits better") to highlighting true differentiators, such as specific service level agreements (e.g., answering calls within 12 seconds) or unique integrated solutions (e.g., specialized PBM wraps for diabetic populations). The conversation heavily emphasizes the evolution of content consumption and the necessity of genuine thought leadership. The speakers agree that traditional marketing collateral (like generic e-brochures posted repeatedly on LinkedIn) is losing efficacy. Instead, the most effective mediums are short-form video content (capturing attention in 6-15 seconds), personalized prospecting (using branded "sales boxes" with personal notes), and authentic communication that reveals the professional's personal values and genuine motivations. This approach, driven by the "decentralization of media," allows professionals to build trust and rapport long before a sales conversation occurs, sometimes resulting in partnerships that materialize years after the initial contact. Looking toward the future, Hinson predicts a continued push toward specialized point solutions (microchasm plug-ins) to solve complex problems (like diabetes management or unique drug savings), stressing that success will hinge not just on the quality of the solution but on the ability to seamlessly implement and integrate these fragmented services into the larger benefit plan. Key Takeaways: • **Regulatory Vigilance is Paramount:** The healthcare and benefits landscape is changing rapidly due to legislation like the Transparency Act. Companies must remain constantly plugged in, as regulatory shifts can fundamentally alter operational requirements overnight (e.g., portal availability, data disclosure requirements). • **The Shift to Self-Funding Complexity:** Moving from fully insured (fixed benefit plans) to self-funded models introduces immense complexity, requiring expertise in cost containment, carve-outs, and vendor integration. Marketing must address this complexity by simplifying the value proposition without losing technical accuracy. • **Jargon Simplification is Essential:** The health benefits industry is rife with acronyms and complex terminology (deductibles, MOOPs, ASO). Effective marketing must translate this jargon into digestible, educational content to shorten the learning curve for buyers and brokers. • **Video is the Dominant Medium:** Video content is the most effective medium for B2B communication in this space, but attention spans are short. Content must be compelling enough to capture the viewer's interest within the first 6 to 15 seconds. • **Personalization Drives Engagement:** Generic marketing collateral is ineffective. Strategies like personalized sales gifts (branded "sales boxes" with personal notes) and customizing collateral to specific industry needs or testimonials applicable to the prospect’s situation significantly increase engagement. • **Thought Leadership Requires Authenticity:** Modern thought leadership involves sharing not just professional expertise but also personal values and motivations. This "personal element" builds trust and rapport, blurring the line between professional and personal life, which became normalized during the pandemic era. • **Decentralization of Solutions:** The future of healthcare cost management involves specialized, best-in-class point solutions (e.g., for diabetes, specialty pharmacy) rather than a single jack-of-all-trades approach. Companies should focus on being "really, really good at one thing." • **Implementation is the Critical Barrier:** While specialized solutions are valuable, the hardest part is often the implementation and integration of these disparate services (widgets) into the existing enterprise infrastructure without overwhelming HR or TPA resources. TPA/vendor roles are becoming increasingly consultative to guide clients through this integration. • **Data Analytics Requires Human Triage:** Big data and analytics are crucial for identifying member needs and offering helpful products. However, the power of data comes from the "synergies" created when human beings triage the data and make meaningful, purposeful decisions based on the insights. • **Long-Term Relationship Building:** Marketing in this industry is not a "one-shot wonder." Consistent effort, constant communication, and providing value over time build "stickiness," often leading to substantial contracts years down the line when the client is finally ready. Key Concepts: * **Decentralization of Healthcare Solutions:** The trend toward unbundling services, moving away from legacy bundled models (like the major carriers) toward specialized, stand-alone point solutions (e.g., specific PBMs, disease management programs) that address narrow, complex problems. * **Table Stakes Marketing:** The basic requirements for marketing in an industry (e.g., having a logo, processing claims efficiently). Effective marketing must move beyond these baseline expectations to highlight unique differentiators. * **Thought Leadership:** Creating substantive content that educates, empowers, and shares expertise, often incorporating personal values to build trust and credibility in advance of a sales conversation. Examples/Case Studies: * **Aortic Valve Replacement Cost Comparison:** Hinson recalled looking at offshore medicine options while at Blue Cross, noting an aortic valve replacement costing $54,000 domestically versus $15,000–$20,000 at a five-star resort-like hospital in Thailand (Bumrungrad), illustrating the dramatic cost differences driving the self-funded market. * **Seven-Year Client Drip:** Ocozzio secured a client after seven years of consistent, low-pressure marketing and relationship-building, demonstrating the necessity of patience and long-term engagement in the complex B2B sales cycle. * **The Placemat Strategy:** Ocozzio helps clients condense extensive 50-page presentations into simple 10-page decks or even single placemats to facilitate conversational selling rather than overwhelming prospects with data.

E3: xEVMPD Maintenance with Donika Doda
The Voice of Life Sciences
/@thevoiceoflifesciences1321
Mar 17, 2022
This video provides an in-depth exploration of xEVMPD (Extended Eudravigilance Medicinal Product Dictionary) maintenance, a critical regulatory compliance requirement for pharmaceutical companies operating within the European Union. Donika Doda, a Regulatory Affairs Specialist, discusses the complexities and strategies involved in making appropriate data submissions to the European Medicines Agency (EMA). The discussion highlights xEVMPD's role as the "mother of EMA databases," established in 2012 as part of Pharmacovigilance Legislation, serving as a central dictionary for medicinal product information and an interface for adverse event reporting. The conversation delves into the practicalities of xEVMPD maintenance, particularly how it fits into the post-approval phase of variation management. When a variation, such as a change to a Summary of Product Characteristics (SPC) or leaflet, is approved and impacts xEVMPD, companies are mandated to submit updated data to Eudravigilance within strict timelines (typically 15 or 30 days). Donika describes a largely manual process where Regulatory Affairs teams receive approved labeling, communicate the change, and then a designated person manually enters the database, finds the correct product, performs the update, and sends it to the EMA, awaiting an acknowledgment message. This manual process is underscored as a legal compliance obligation, crucial for ensuring healthcare professionals have access to the most current and accurate information, especially concerning safety variations. A significant portion of the discussion focuses on the challenges associated with xEVMPD maintenance. These include the inherent complexity of manual data entry, the need for precise timing, and the organizational hurdles arising from involving multiple departments like Pharmacovigilance and Regulatory Affairs. Donika emphasizes that the number of steps in the process directly impacts efficiency, advocating for streamlined workflows. A major pain point identified is the pervasive data duplication across various departmental software systems that often "do not talk to each other," leading to inefficiencies and wasted resources. This fragmentation necessitates a broader industry shift towards digital transformation. Looking to the future, the video introduces IDMP (Identification of Medicinal Products) as the "big brother" to xEVMPD, representing a significant opportunity for the industry to enhance efficiency and collaboration. IDMP is anticipated to facilitate a real interface between company software systems and the EMA, drastically reducing the time and effort required for data communication and maintenance. This upcoming standard is seen as a catalyst for companies to align their data, foster inter-departmental collaboration, and reduce duplication. The speakers advocate for companies to embrace this digital transformation, potentially by establishing dedicated teams responsible for managing this transition, ultimately leading to quicker access to reliable information for patients, healthcare professionals, and more efficient assessment processes for regulators. Key Takeaways: * **xEVMPD as a Core Regulatory Database:** The Extended Eudravigilance Medicinal Product Dictionary (xEVMPD) serves as the foundational "mother of EMA databases," centralizing medicinal product information and linking to pharmacovigilance for adverse event reporting. * **Mandatory Compliance for EU Operations:** Since 2012, xEVMPD maintenance has been a mandatory legal compliance requirement for all pharmaceutical companies with products registered in the European Union. * **Manual and Time-Sensitive Update Process:** Following the approval of variations (e.g., changes to SPC or leaflets), companies must manually update xEVMPD data within strict deadlines (15 or 30 days), involving regulatory affairs teams and direct communication with the EMA. * **Critical for Patient Safety and Healthcare Professionals:** Maintaining an up-to-date xEVMPD database is vital for patient safety, as healthcare professionals rely on this information from their respective authorities to ensure they have the latest details on prescribed or used products, especially for safety variations. * **Challenges of Inter-Departmental Complexity:** The maintenance process is complicated by involving multiple departments, such as Pharmacovigilance and Regulatory Affairs, highlighting the need for clear roles, responsibilities, and efficient communication channels. * **Inefficiencies Due to Data Duplication and Disparate Systems:** A significant challenge is the widespread duplication of information across different departmental software systems that lack interoperability, leading to wasted resources and increased effort. * **IDMP as a Catalyst for Digital Transformation:** The upcoming IDMP (Identification of Medicinal Products) standard is viewed as a major opportunity to drive digital transformation, promising a real interface between company software and the EMA, thereby reducing communication and maintenance times. * **Opportunity for Streamlined Operations and Collaboration:** IDMP implementation encourages companies to align data, foster greater collaboration between departments, and reduce data duplication, leading to more efficient internal operations. * **Need for Dedicated Digital Transformation Teams:** Companies are advised to consider establishing dedicated teams responsible for managing digital transformation initiatives to ensure a cohesive approach and successful adoption of new technologies and regulatory standards. * **Benefits of Digital Adoption:** Embracing digital transformation and implementing connectors between systems will reduce timing and effort, improve data reliability, and ultimately lead to quicker access to information for patients and healthcare professionals, as well as more efficient regulatory assessments. Key Concepts: * **xEVMPD (Extended Eudravigilance Medicinal Product Dictionary):** A mandatory EMA database containing information on all medicinal products registered in the European Union, serving as a central dictionary and linked to pharmacovigilance. * **Eudravigilance:** The European Medicines Agency's (EMA) system for managing and analyzing information on suspected adverse reactions to medicines authorized in the European Economic Area (EEA). * **EMA (European Medicines Agency):** The agency responsible for the scientific evaluation, supervision, and safety monitoring of medicines in the European Union. * **Pharmacovigilance:** The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem. * **IDMP (Identification of Medicinal Products):** A set of ISO standards for the unique identification of medicinal products, aimed at standardizing the way medicines are identified and described globally. * **SPC (Summary of Product Characteristics):** A document that describes the properties and the approved conditions of use of a medicinal product. * **Leaflet:** The patient information leaflet (PIL) that comes with a medicine, providing information on how to use it safely and effectively. * **Variation Management:** The process of managing changes to a medicinal product's marketing authorization after it has been approved. Tools/Resources Mentioned: * **Eudravigilance:** The database to which xEVMPD updates are submitted. * **Vault:** Mentioned as a potential platform where connectors might be built for future integration.

The Medium is the Message
Self-Funded
@SelfFunded
Mar 15, 2022
This video segment explores the critical role of communication mediums in marketing effectiveness, centering on the concept coined by Marshall McLuhan: "The medium is the message." The discussion, featuring Trey Hinson, COO of Ocozzio, emphasizes that the format and channel through which a message is delivered often dictate its success, especially in today's saturated digital environment. The core argument presented is that marketers must prioritize mediums that capture fleeting attention spans and personalize content to break through the noise, moving away from outdated or generic collateral. The speakers identify video as the single most effective medium currently available for content digestion. They note that audiences are willing to engage with video content for short bursts—typically 6 to 15 seconds—but rarely longer. This preference highlights the need for concise, impactful messaging delivered through dynamic formats. Beyond digital mediums, the conversation stresses the power of personalized physical outreach, such as branded sales boxes containing a personalized note and relevant items. This approach leverages the human desire for recognition and appreciation, making the recipient feel valued ("thank you for thinking about me"), which greatly enhances the likelihood of engagement compared to mass-produced materials. A significant portion of the analysis focuses on the challenges of B2B communication on platforms like LinkedIn. The speakers observe that LinkedIn has developed a "scrolling effect," similar to other social platforms, where users quickly bypass content that doesn't immediately provide a hook. This environment renders traditional marketing collateral—such as generic pamphlets, e-brochures, or embedded PDFs—largely ineffective unless they contain a "riveting stat" or immediate, actionable value. Furthermore, the increasing prevalence of sponsored posts (noting that 1 in 4 to 1 in 6 posts in a typical feed is now an ad) is desensitizing users to standard marketing messages. To counteract this, the speakers advocate for highly personalized content, including incorporating personal elements (e.g., family pictures) alongside professional messages, to create a necessary "antithesis" to the constant influx of commercial content and build trust with the audience. The underlying principle is that people want to know who they are doing business with, making the blurring of professional and personal content a strategic advantage. Key Takeaways: • **Video is the Dominant Medium for Attention:** Audiences are overwhelmingly digesting content via video, but attention spans are extremely short, typically ranging from 6 to 15 seconds. Marketing messages must be designed to deliver maximum impact within this brief window. • **Avoid Generic Collateral on Digital Platforms:** Traditional marketing materials like PDFs, e-brochures, and generic flyers posted on LinkedIn are often ignored because users are desensitized to standard commercial content and do not want to click on embedded documents unless immediate, high-value data is promised. • **Personalization Drives Engagement:** Customization is paramount. Whether through video or physical outreach, tailoring the message or collateral specifically to the individual prospect significantly increases the likelihood of them reading, watching, or responding to the communication. • **Leverage Personalized Physical Gifts:** Sending personalized sales gifts, such as a branded box with a personal note, creates a powerful, positive impression. This method provides a tangible "thank you" that fosters goodwill and makes the recipient feel appreciated, enhancing the relationship beyond a digital transaction. • **The LinkedIn Scrolling Effect Requires a Strong Hook:** Due to the rapid scrolling behavior on LinkedIn, content must instantaneously grab attention. If the message doesn't hook the viewer immediately, they will bypass it, making the initial presentation critical for success. • **Sponsored Posts are Desensitizing Audiences:** The high volume of sponsored content (estimated at 1 in 4 to 1 in 6 posts) is making users less receptive to traditional advertisements. This necessitates more creative, non-standard approaches to paid promotion to stand out. • **Test and Collect Data on Medium Effectiveness:** Marketers should continuously test different mediums and content styles (e.g., personal vs. professional posts) and collect data to determine what truly resonates with their specific target audience, rather than relying on assumptions about platform best practices. • **Integrate Personal Elements into Professional Messaging:** To cut through the noise and build trust, incorporating personal context (e.g., a message underlying a picture of family) can serve as the initial hook that stops the scroll, allowing the professional value proposition to be delivered effectively. • **Medium Appropriateness is Key:** Not all mediums are suitable for every message. A complex value proposition should not be delivered via a medium designed for quick consumption (like a LinkedIn PDF) unless the content is highly condensed and immediately valuable. Tools/Resources Mentioned: * LinkedIn (as a marketing platform) * Sales Box (a concept for personalized physical sales gifts) Key Concepts: * **The Medium is the Message:** A communication theory stating that the form of a medium embeds itself in the message, creating a symbiotic relationship by which the medium influences how the message is perceived. * **Scrolling Effect:** The observed behavior on social media platforms where users rapidly scroll past content, demanding that posts capture attention instantly to be noticed. * **Sponsored Posts:** Paid advertisements integrated into a social media feed, which, due to their high volume, are contributing to audience desensitization.

Behavior Modification in Healthcare
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Mar 12, 2022
This video provides an in-depth exploration of behavior modification in healthcare, leveraging Professor BJ Fogg's renowned model. Dr. Eric Bricker, the presenter, begins by establishing the fundamental premise that improving health outcomes, quality, and reducing costs necessitates a change in human behavior. The core of the discussion revolves around applying Fogg's model—which posits that behavior change requires the simultaneous presence of Motivation, Ability, and a Trigger—to increase primary care utilization among members of employer-sponsored health plans. The presentation meticulously breaks down each component of the Fogg model, illustrating how a deficiency in any one area can prevent desired behavior change. Dr. Bricker uses a visual representation of Fogg's "action line," explaining that for difficult tasks, high motivation is required, while for easy tasks, low motivation suffices. Crucially, triggers only work when an individual is on the "right side" of this action line, meaning they possess sufficient motivation and ability. If a task is too hard or motivation is too low, triggers will fail, regardless of how frequently they are applied. Dr. Bricker then applies this framework to the real-world problem of low primary care engagement, citing statistics where only 15-50% of employees in large companies see a primary care physician within two years. He analyzes the current state, identifying that motivation is often low because the body is remarkably resilient, masking early signs of disease, and job-based incentives often fall flat due to general employee disengagement. Ability is severely hampered by the logistical difficulties of traditional primary care (time off work, travel, waiting, short doctor visits). Triggers are ineffective because they are typically generic and not personalized to individual needs, such as a 25-year-old male versus a 45-year-old female. The video concludes by outlining solutions for each component, drawing on the ancient Greek principles of persuasion (ethos, pathos, logos) for motivation, modern access solutions (on-site, virtual primary care) for ability, and personalized, multi-channel communication for triggers. The discussion culminates with a compelling case study of Serigraph, an automotive parts manufacturer that successfully kept its healthcare costs flat for nearly a decade by implementing these behavior modification strategies. Serigraph utilized a combination of monetary and time-off incentives, established an on-site clinic to drastically improve access and ease (ability), and employed "hyper-communication" through various channels, including mandated annual face-to-face coaching, to deliver highly personalized triggers. This real-world example underscores the practical applicability and profound impact of Fogg's model when systematically applied to healthcare challenges. Key Takeaways: * **BJ Fogg's Behavior Model (B=MAT):** Behavior change (B) is a product of Motivation (M), Ability (A), and a Trigger (T). All three elements must be present simultaneously for a behavior to occur. * **The "Action Line":** Fogg's model includes an "action line" on a graph where motivation (y-axis) and ability (x-axis) intersect. Behaviors above this line are more likely to occur with a trigger; those below will not, regardless of triggers. * **Motivation Challenges in Healthcare:** Individuals often lack motivation for preventative health due to the body's ability to mask disease symptoms until advanced stages. Generic job-based health incentives frequently fail to engage employees. * **Ability Barriers in Traditional Primary Care:** Accessing traditional primary care is often difficult, requiring significant time off work, travel, and waiting, making it a "half-day escapade" for a brief doctor interaction. This high effort severely limits ability. * **Ineffective Triggers:** Broad, non-personalized communication (e.g., mass emails) fails to resonate with diverse employee populations, as healthcare needs vary significantly by age, gender, and other demographics. * **Building Motivation through Persuasion:** Effective motivation for health comes from credible sources (doctors - Ethos), empathetic listening (Pathos), and then logical reasoning (Logos). Starting with logic without credibility and empathy is ineffective. * **Enhancing Ability with Accessible Care:** Making primary care "super easy" is crucial, especially for those with low motivation. Solutions include on-site clinics, near-site clinics, direct primary care, and virtual primary care, which reduce logistical barriers. * **Personalized Triggers are Essential:** Communication must be customized to the individual's needs and preferences. This can involve different channels (emails, texts, hard copies, spousal communication) and face-to-face coaching for tailored messaging. * **Triggers Fail Below the Action Line:** If motivation is low and ability is hard (below the action line), no amount of triggering (emails, texts, counseling) will result in behavior change; the foundational issues of motivation and ability must be addressed first. * **Serigraph Case Study - Integrated Approach:** The manufacturing company Serigraph successfully flattened healthcare costs for 10 years by combining incentives (monetary, time off), an on-site clinic (improving ability), and "hyper-communication" including mandated annual face-to-face coaching (personalized triggers). * **The Power of On-Site Clinics:** For concentrated workforces, on-site clinics significantly enhance ability by making care convenient, quick, and requiring no time off, which was a "crux" of Serigraph's success. * **Virtualization for Distributed Workforces:** For companies with geographically dispersed employees, virtual primary care is critical to replicate the "ease" factor of on-site clinics and overcome ability barriers. * **Conversations as Key to Customization:** Face-to-face coaching allows for highly customized triggers, as coaches can listen, empathize, and tailor their message based on individual conversations, addressing specific social determinants of health. * **Hyper-Communication Strategy:** Serigraph's success involved utilizing a multitude of communication channels—in-person meetings, emails, flyers, HR staff, managers—to ensure messages reached employees and their families effectively. Key Concepts: * **BJ Fogg's Behavior Model (B=MAT):** A framework stating that behavior change occurs when motivation, ability, and a trigger converge simultaneously. * **Action Line:** A graphical representation within Fogg's model indicating the threshold of motivation and ability required for a trigger to be effective. * **Ethos, Pathos, Logos:** Ancient Greek principles of persuasion: Ethos (credibility), Pathos (empathy), Logos (logic). The video emphasizes the importance of establishing ethos and pathos before presenting logos in healthcare communication. Examples/Case Studies: * **Serigraph:** An automotive parts manufacturer in Wisconsin that kept its healthcare costs flat for almost 10 years. Their strategy included monetary and time-off incentives, an on-site primary care clinic, and extensive, personalized communication including mandated annual face-to-face coaching.
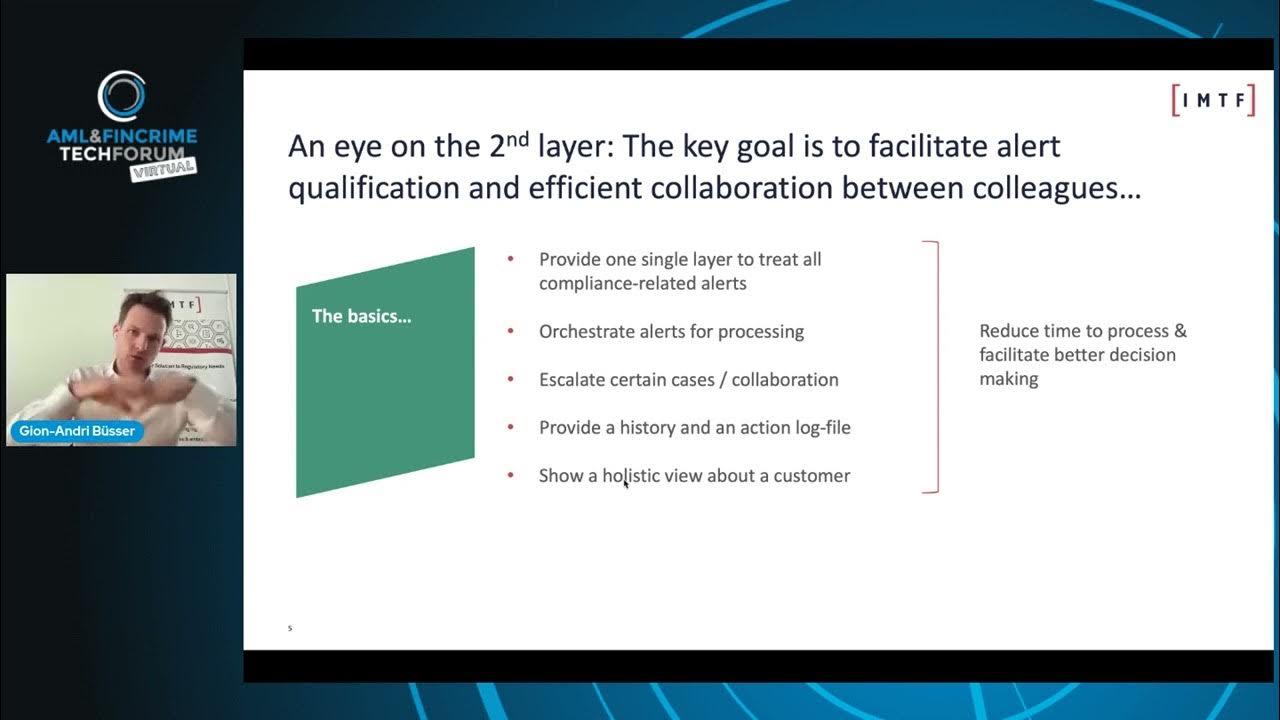
Benefits of a Two Layer Regulatory Intelligence Platform
IMTF - Excellence in RegTech Solutions
/@imtf-excellenceinregtech
Mar 11, 2022
The speaker, Gion-Andri Büsser of IMTF, discusses the critical need for modern technology in compliance, driven by exploding costs of compliance and even higher costs of non-compliance. He introduces a "two-layer regulatory intelligence platform" concept, emphasizing an orchestration layer that centralizes the treatment of compliance alerts, automates processes, fosters collaboration, and provides a holistic view of data. The video highlights how advanced technologies, including machine learning and data science, can be leveraged within this architecture to significantly reduce manual work, improve efficiency, and enhance risk management. Key Takeaways: * **Ecosystem Approach to Compliance Technology:** The video advocates for viewing compliance technology as an integrated ecosystem rather than disparate, siloed software packages. This platform approach is crucial for comprehensive data utilization and decision-making. * **The Power of the Orchestration Layer (Layer 2):** A dedicated orchestration layer is presented as essential for consolidating and processing all compliance alerts (e.g., AML, fraud, sanctions), enabling flexible process automation, fostering collaboration across teams, and maintaining accurate, regulator-ready audit trails. * **AI/ML for Enhanced Efficiency and Risk Reduction:** The "magic" of the orchestration layer lies in its ability to leverage AI and machine learning for advanced functions such as intelligent auto-closure of routine alerts, pre-processing to highlight historical patterns, and more granular, accurate risk rating based on comprehensive customer history. * **Strategic IT and Change Management Benefits:** Implementing an orchestration layer provides significant IT flexibility, allowing organizations to introduce modern tools and interfaces without immediately replacing existing, potentially valuable, legacy detection systems (Layer 1). This enables a phased modernization approach, reducing disruption and risk. * **Continuous Adaptation to Evolving Threats:** The speaker underscores that financial crime and compliance challenges are an "ever-ending battle," with criminal behaviors constantly evolving (e.g., due to pandemics, crypto). This necessitates adaptive, forward-looking technology to anticipate and combat new threats.g., GxP, 21 CFR Part 11, FDA/EMA regulations).